��Ŀ����
����Ŀ��
�������漰�л��������˵������ȷ���� ______________________
A����ȥ��������������ϩ��ͨ������KMnO4��Һ���з���
B���ױ������ƶ������ױ��뱽������Ҵ���Ӧ�Ʊ����������ķ�Ӧ���Ͳ�ͬ
C��������������Һ�������ͺ�����
D����ȥ�Ҵ��������������������ʯ�ң�����
E����ȥ��������������������ñ�������������Һϴ�ӡ���Һ���������
������ȩ��������õ�����������ȩ��1-������Ʒ��Ϊ����1-��������С���������֪����R-CHO+NaHSO3�����ͣ���RCH��OH��SO3Na����
�ڷе㣺����34�棬1-����118�棬����Ƴ������ᴿ·�ߣ�
![]()
��1���Լ�1Ϊ__________������2Ϊ__________������3Ϊ__________��
��2��д������ȩ������Ӧ����ʽ___________________________________
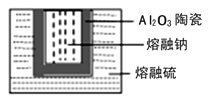
����֪��1,2-��������������Ϳ����������Ӽ�,������������ɫҺ��,�ܶ���2.18��/����3,�е�131.4��,�۵�9.79��,������ˮ,�����ڴ����ѡ���ͪ���л��ܼ�����ʵ���п�������ͼ��ʾװ���Ʊ�1,2-�������顣���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ,�Թ�d��װ��Һ��(���渲������ˮ���������ж�)������д���пհף�
��1��д���Ʊ�1,2-��������Ļ�ѧ����ʽ����________________________________��
��2����ȫƿb���Է�ֹ����,�����Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����_______________________________________��
��3��cװ����NaOH��Һ��������______________________________��
��4��eװ����NaOH��Һ��������_______________________________��
���𰸡� CD ����NaHSO3��Һ ��ȡ ���� CH3CH2CH2CHO+2Ag(NH3)2OH![]() CH3CH2CH2COONH4+2Ag��+3NH3+H2O CH2��CH2��Br2 �� CH2BrCH2Br b��ˮ����½����������е�ˮ���������������� �����������壨������������̼�ȣ� ���ջӷ��������壬��ֹ��Ⱦ������
CH3CH2CH2COONH4+2Ag��+3NH3+H2O CH2��CH2��Br2 �� CH2BrCH2Br b��ˮ����½����������е�ˮ���������������� �����������壨������������̼�ȣ� ���ջӷ��������壬��ֹ��Ⱦ������
������������A������KMnO4��Һ�ܰ���ϩ����ΪCO2���������ʣ�Ӧ������ˮ��A������B���ױ������ƶ������ױ��뱽������Ҵ���Ӧ�Ʊ����������ķ�Ӧ������ͬ������ȡ����Ӧ��B������C��������������֬�������������Ʒ���������Ӧ�������������࣬��˿���������������Һ�������ͺ�������C��ȷ��D������������Ʒ�Ӧ����˳�ȥ�Ҵ��������������������ʯ�ң�������D��ȷ��E�����������ܺ�����������Ӧ����ȥ��������������������ñ���̼������Һϴ�ӡ���Һ�����������E����ѡCD��
������1����Ʒ�к�������ȩ��������������Ϣ���ñ���NaHSO3��Һ�γɳ�����Ȼ��ͨ�����˼��ɳ�ȥ�����ڱ���NaHSO3��Һ�ǹ����ģ����Լ������ѵ�Ŀ������ȡ��Һ�е�1-��������Ϊ1-���������ѵķе����ܴ���˿���������������뿪����2������ȩ����������Ӧ����ʽΪCH3CH2CH2CHO+2Ag(NH3)2OH![]() CH3CH2CH2COONH4+2Ag��+3NH3+H2O��
CH3CH2CH2COONH4+2Ag��+3NH3+H2O��
������1����ϩ����ˮ�����ӳɷ�Ӧ����1��2�������飬��ѧ����ʽΪCH2��CH2��Br2 �� CH2BrCH2Br����2�� ��d�з��������������ɵ��������ų�������b��ѹǿ��������b��ˮ����½����������е�ˮ��������������������3��Ũ���������ˮ�Ժ�ǿ�����ԣ��ڼ��ȵ�����£��Ҵ���Ũ���ᷢ������Ӧ���ɶ�����̼������������������壬��������������ˮ��Ӧ�������ᡢ�����ᡣΪ�˷�ֹ���Ʊ�1��2����������ĸ��ţ�����c��NaOH��Һ�������dz�ȥ��ϩ�д������������壻��4��Һ���ӷ����ƵõIJ����к����ж�������������eװ����NaOH��Һ�����������ջӷ��������壬��ֹ��Ⱦ������

����Ŀ������2 L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)��O2(g) ![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
N(NO)(mol) | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
(1)800�棬��Ӧ�ﵽƽ��ʱ��NO�����ʵ���Ũ����________________��
(2)��ͼ�б�ʾNO2�ı仯��������____________��
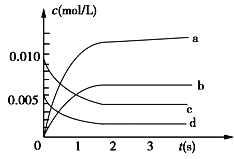
(3)��˵���÷�Ӧ�Ѵﵽƽ��״̬����____________��
a��v(NO2)��2v(O2) b��������ѹǿ���ֲ���
c��v��(NO)��2v��(O2) d���������ܶȱ��ֲ���
(4)��ʹ�÷�Ӧ�ķ�Ӧ�����������____________��
a����ʱ�����NO2���� b���ʵ������¶�
c�������������ݻ� d��ѡ���Ч����
��Ǧ�����dz��õĻ�ѧ��Դ����缫������Pb��PbO2�����ҺΪϡ���ᡣ����ʱ�õ���ܷ�ӦʽΪPbO2��Pb��2H2SO4===2PbSO4��2H2O���ݴ��жϣ�
��1��Ǧ���صĸ���������____________��
��2������ʱ���������Һ������____________(���������С�����䡱)��
��3������ʱ���������Һ������������____________����
��4�����·�У����������____________������____________����