��Ŀ����
��1�����������Ҫ�ɷ���Al2O3��3H2O������������Fe2O3��д�����������Ʊ��������ĸ�����Ļ�ѧ����ʽ����2013��߿���Ƭ�Σ�Դ�ڸ�һ�̲ģ�
��2��������ԭ���г�����������ΪSiO2������̿��ʯ��ʯ���豸Ϊ��¯��д������ʱ����������Ҫ�Ļ�ѧ��Ӧ����ʽ
��3����ҵ����ʯӢɰ��������ȡ�ֹ裬�����ԭ��Ӧ��ȡ�ߴ��裬�䷴Ӧ����ʽ�ֱ�Ϊ��
��4������û��Fe�������
����û��Al�������
����û��Si�������
��2��������ԭ���г�����������ΪSiO2������̿��ʯ��ʯ���豸Ϊ��¯��д������ʱ����������Ҫ�Ļ�ѧ��Ӧ����ʽ
��3����ҵ����ʯӢɰ��������ȡ�ֹ裬�����ԭ��Ӧ��ȡ�ߴ��裬�䷴Ӧ����ʽ�ֱ�Ϊ��
��4������û��Fe�������
����û��Al�������
����û��Si�������
��27�֣�����ʽ��2�֣���4���ʹ�3��
��1����Al2O3+2NaOH��2NaAlO2+H2O ��NaAlO2+CO2+2H2O��Al(OH)3��+NaHCO3
��2Al(OH)3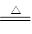 Al2O3+3H2O ��2Al2O3
Al2O3+3H2O ��2Al2O3 4Al+3 O2��
4Al+3 O2��
��2����C+O2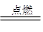 CO2 ��CO2+C
CO2 ��CO2+C 2CO ��Fe2O3+3CO
2CO ��Fe2O3+3CO 2Fe+3CO2
2Fe+3CO2
��CaCO3 CaO+CO2�� ��CaO+SiO2
CaO+CO2�� ��CaO+SiO2 CaSiO3
CaSiO3
��3����SiO2+2C Si���ֹ裩+2CO�� ��Si+2Cl2
Si���ֹ裩+2CO�� ��Si+2Cl2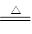 SiCl4
SiCl4
��SiCl4+2H2===Si���ߴ��裩+4HCl
��4�������𰸶����֡�û��Fe����ԭʼ�˵����û��Al�����⽨������û��Si��û������Ϣʱ�������
��1����Al2O3+2NaOH��2NaAlO2+H2O ��NaAlO2+CO2+2H2O��Al(OH)3��+NaHCO3
��2Al(OH)3
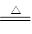 Al2O3+3H2O ��2Al2O3
Al2O3+3H2O ��2Al2O3 4Al+3 O2��
4Al+3 O2����2����C+O2
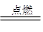 CO2 ��CO2+C
CO2 ��CO2+C 2CO ��Fe2O3+3CO
2CO ��Fe2O3+3CO 2Fe+3CO2
2Fe+3CO2��CaCO3
 CaO+CO2�� ��CaO+SiO2
CaO+CO2�� ��CaO+SiO2 CaSiO3
CaSiO3��3����SiO2+2C
 Si���ֹ裩+2CO�� ��Si+2Cl2
Si���ֹ裩+2CO�� ��Si+2Cl2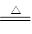 SiCl4
SiCl4��SiCl4+2H2===Si���ߴ��裩+4HCl
��4�������𰸶����֡�û��Fe����ԭʼ�˵����û��Al�����⽨������û��Si��û������Ϣʱ�������
�����������1�����������Ҫ�ɷ���Al2O3��3H2O��������Fe2O3�����ᴿ������ʱ�����ȥ�����������������������������֪�����������ܽ�������������Һ�У��������ܽ⣬����ƫ�����ƣ����˵õ�ƫ��������Һ����ƫ��������ͨ��CO2�����������������������˼��õ��������������������ֽ�������������������������õ���������
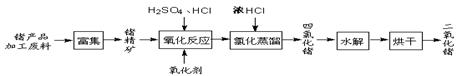
��2����¯����ʱ��̿ȼ������CO2��������̼��CO2��Ӧ������CO�����ɵ�CO���л�ԭ�ԣ��ڸ����¿��Ի�ԭ���������ڸ�����ʯ��ʯ�ֽ����������ƺ�CO2�����ɵ��������ڸ������ںͶ������跴Ӧ���ɹ���ơ�
��3��ʯӢɰ����Ҫ�ɷ��Ƕ������裬�ڸ����ºͶ������跴Ӧ���ɴֹ衣�ֹ�������ڸ����·�Ӧ�������Ȼ��裬Ȼ������������ԭ���Ȼ����û������ʹ衣
�����������ǻ���������Ŀ��飬���������ǿ�����۽̲Ļ���֪ʶ�������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ�����ԡ�Ҳ����������ѧ���������������淶�Ĵ���������

��ϰ��ϵ�д�
�����Ŀ
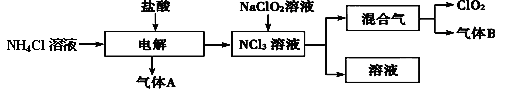
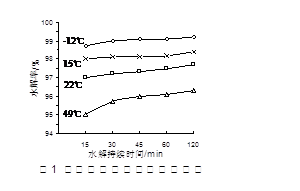
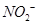 �������ڼ��������¼������۳�ȥ�����ȴ�����ķ�ˮ�������ʹʪ��ĺ�ɫʯ����ֽ���������壩����ȥ
�������ڼ��������¼������۳�ȥ�����ȴ�����ķ�ˮ�������ʹʪ��ĺ�ɫʯ����ֽ���������壩����ȥ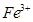
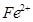
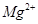
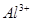
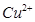
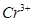
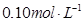 ��������ϱ����������ݺ��ṩ��ҩƷ��������ȥCuCl2��Һ��
��������ϱ����������ݺ��ṩ��ҩƷ��������ȥCuCl2��Һ��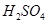 ��NaOH��Һ��CuO��Cu����
��NaOH��Һ��CuO��Cu����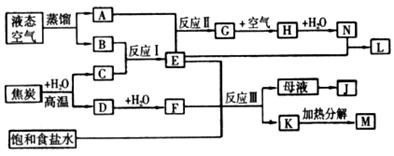
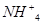 ��Ũ�ȣ�ʹJ���������
��Ũ�ȣ�ʹJ���������
 2Na��Cl2��
2Na��Cl2�� 2Al��3H2O
2Al��3H2O 2Hg��O2��
2Hg��O2��