��Ŀ����
��8�֣�Q��R��X��Y��Z����Ԫ�ص�ԭ���������ε�������֪�� ��Z��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ�
��Z��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ� ��Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�msnmpn
��Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�msnmpn  ��Rԭ�Ӻ���L�������Ϊ������
��Rԭ�Ӻ���L�������Ϊ������ ��Q��Xԭ��p����ĵ������ֱ�Ϊ2��4��
��Q��Xԭ��p����ĵ������ֱ�Ϊ2��4�� ��ش��������⣺
��ش��������⣺
 ��1��Z2+ �ĺ�������Ų�ʽ�� ��Zԭ�ӻ�̬ʱ�����Ų�ʽΪ ��
��1��Z2+ �ĺ�������Ų�ʽ�� ��Zԭ�ӻ�̬ʱ�����Ų�ʽΪ ��
 ��2��Q��Y�γɵ������̬�⻯��ֱ�Ϊ�ס��ң������ж���ȷ���� ��
��2��Q��Y�γɵ������̬�⻯��ֱ�Ϊ�ס��ң������ж���ȷ���� ��

 ��3��Q��R��Y����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ ����Ԫ�ط������𣩡�
��3��Q��R��Y����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ ����Ԫ�ط������𣩡�
 ��4������Ԫ���У��縺���������С�����ַǽ���Ԫ���γɵĻ�����Ļ�ѧʽΪ ��
��4������Ԫ���У��縺���������С�����ַǽ���Ԫ���γɵĻ�����Ļ�ѧʽΪ ��
 ��5��Q��һ���⻯����Է�������Ϊ28�����з����еĦҼ���м��ļ���֮��Ϊ �����⻯���������γɵ�ȼ�ϵ���ڼ��Ե�����и�����ӦΪ ��
��5��Q��һ���⻯����Է�������Ϊ28�����з����еĦҼ���м��ļ���֮��Ϊ �����⻯���������γɵ�ȼ�ϵ���ڼ��Ե�����и�����ӦΪ ��
 ��Z��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ�
��Z��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ� ��Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�msnmpn
��Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�msnmpn  ��Rԭ�Ӻ���L�������Ϊ������
��Rԭ�Ӻ���L�������Ϊ������ ��Q��Xԭ��p����ĵ������ֱ�Ϊ2��4��
��Q��Xԭ��p����ĵ������ֱ�Ϊ2��4�� ��ش��������⣺
��ش��������⣺ ��1��Z2+ �ĺ�������Ų�ʽ�� ��Zԭ�ӻ�̬ʱ�����Ų�ʽΪ ��
��1��Z2+ �ĺ�������Ų�ʽ�� ��Zԭ�ӻ�̬ʱ�����Ų�ʽΪ �� ��2��Q��Y�γɵ������̬�⻯��ֱ�Ϊ�ס��ң������ж���ȷ���� ��
��2��Q��Y�γɵ������̬�⻯��ֱ�Ϊ�ס��ң������ж���ȷ���� ��
| A���ȶ��ԣ���>�ң��е㣺��>�� | B���ȶ��ԣ���>�ң��е㣺�ף��� | C���ȶ��ԣ���<�ң��е㣺��<�� | D���ȶ��ԣ���<�ң��е㣺��>�� |
 ��3��Q��R��Y����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ ����Ԫ�ط������𣩡�
��3��Q��R��Y����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ ����Ԫ�ط������𣩡� ��4������Ԫ���У��縺���������С�����ַǽ���Ԫ���γɵĻ�����Ļ�ѧʽΪ ��
��4������Ԫ���У��縺���������С�����ַǽ���Ԫ���γɵĻ�����Ļ�ѧʽΪ �� ��5��Q��һ���⻯����Է�������Ϊ28�����з����еĦҼ���м��ļ���֮��Ϊ �����⻯���������γɵ�ȼ�ϵ���ڼ��Ե�����и�����ӦΪ ��
��5��Q��һ���⻯����Է�������Ϊ28�����з����еĦҼ���м��ļ���֮��Ϊ �����⻯���������γɵ�ȼ�ϵ���ڼ��Ե�����и�����ӦΪ ����1��1s22s22p63s23p63d9 [Ar]3d104s1 ��2��B ��3��Si<C<N
��4��SiO2 ��5��5:1��C2H4 - 12e- +16OH- =2CO32-+ 10H2O
��4��SiO2 ��5��5:1��C2H4 - 12e- +16OH- =2CO32-+ 10H2O
Z��ԭ������Ϊ29����Z��ͭԪ�ء�Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�msnmpn ����Y�ǵڢ�AԪ�أ�Q��Xԭ��p����ĵ������ֱ�Ϊ2��4������ΪQ��R��X��Y��Z����Ԫ�ص�ԭ���������ε���������Q��C��X��O������Rԭ�Ӻ���L�������Ϊ����������L��N����Y��Si��
����Y�ǵڢ�AԪ�أ�Q��Xԭ��p����ĵ������ֱ�Ϊ2��4������ΪQ��R��X��Y��Z����Ԫ�ص�ԭ���������ε���������Q��C��X��O������Rԭ�Ӻ���L�������Ϊ����������L��N����Y��Si��
��1�����ݹ���ԭ����֪��ͭ���ӵĺ�������Ų�ʽ��1s22s22p63s23p63d9����ͭԭ�ӻ�̬ʱ�����Ų�ʽΪ[Ar]3d104s1��
��2���ǽ�����Խǿ���⻯����ȶ���Խǿ��̼Ԫ�صķǽ�����ǿ�ڹ�Ԫ�صģ�����̼���⻯���ȶ���ǿ�ڹ�Ԫ�ص��⻯�����Ϊ���ߵ��⻯���γɵľ��嶼�Ƿ��Ӿ��壬�е�ߵ�����Ӽ���������С�й�ϵ����˼���ķе����SiH4�ķе㣬��ѡB��
��3���ǽ�����Խǿ����һ������Խ�ǽ�������Si<C<N�����Ե�һ��������Si<C<N��
��4���ǽ�����Խǿ�縺��Խ�����Ե縺����������Ԫ�أ���С�ķǽ���Ԫ���ǹ�Ԫ�أ������γɵĻ������Ƕ������裬��ѧʽ��SiO2��
��5��̼Ԫ�ص��⻯������Է���������28�Ļ���������ϩ��������ϩ�����к���̼̼˫������˫������1���Ҽ���1���м����ɵģ��������ǦҼ���������ϩ�����еĦҼ���м��ļ���֮��Ϊ5:1��ԭ����и���ʧȥ���ӣ������õ����ӣ�������ϩ�ڸ���ͨ�룬����������ͨ�롣����Ϊ�������Һ�Լ��ԣ����Ը����缫��Ӧʽ��C2H4 - 12e- +16OH- =2CO32-+ 10H2O��
 ����Y�ǵڢ�AԪ�أ�Q��Xԭ��p����ĵ������ֱ�Ϊ2��4������ΪQ��R��X��Y��Z����Ԫ�ص�ԭ���������ε���������Q��C��X��O������Rԭ�Ӻ���L�������Ϊ����������L��N����Y��Si��
����Y�ǵڢ�AԪ�أ�Q��Xԭ��p����ĵ������ֱ�Ϊ2��4������ΪQ��R��X��Y��Z����Ԫ�ص�ԭ���������ε���������Q��C��X��O������Rԭ�Ӻ���L�������Ϊ����������L��N����Y��Si����1�����ݹ���ԭ����֪��ͭ���ӵĺ�������Ų�ʽ��1s22s22p63s23p63d9����ͭԭ�ӻ�̬ʱ�����Ų�ʽΪ[Ar]3d104s1��
��2���ǽ�����Խǿ���⻯����ȶ���Խǿ��̼Ԫ�صķǽ�����ǿ�ڹ�Ԫ�صģ�����̼���⻯���ȶ���ǿ�ڹ�Ԫ�ص��⻯�����Ϊ���ߵ��⻯���γɵľ��嶼�Ƿ��Ӿ��壬�е�ߵ�����Ӽ���������С�й�ϵ����˼���ķе����SiH4�ķе㣬��ѡB��
��3���ǽ�����Խǿ����һ������Խ�ǽ�������Si<C<N�����Ե�һ��������Si<C<N��
��4���ǽ�����Խǿ�縺��Խ�����Ե縺����������Ԫ�أ���С�ķǽ���Ԫ���ǹ�Ԫ�أ������γɵĻ������Ƕ������裬��ѧʽ��SiO2��
��5��̼Ԫ�ص��⻯������Է���������28�Ļ���������ϩ��������ϩ�����к���̼̼˫������˫������1���Ҽ���1���м����ɵģ��������ǦҼ���������ϩ�����еĦҼ���м��ļ���֮��Ϊ5:1��ԭ����и���ʧȥ���ӣ������õ����ӣ�������ϩ�ڸ���ͨ�룬����������ͨ�롣����Ϊ�������Һ�Լ��ԣ����Ը����缫��Ӧʽ��C2H4 - 12e- +16OH- =2CO32-+ 10H2O��

��ϰ��ϵ�д�
�����Ŀ
 ������Ӳ�����ͬ�������������ǿ�ᷴӦ�����ӷ���ʽΪ________________________________��2�֣�������������Ӧˮ�������������ط�Ӧ�Ļ�ѧ����ʽΪ _________________________________________��2�֣���
������Ӳ�����ͬ�������������ǿ�ᷴӦ�����ӷ���ʽΪ________________________________��2�֣�������������Ӧˮ�������������ط�Ӧ�Ļ�ѧ����ʽΪ _________________________________________��2�֣���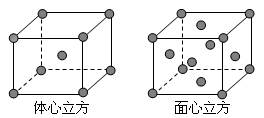
 �����������2��1��ȫ��Ӧ���������ڳ�������Һ�塣��Һ����D�����ܾ��ҷ�Ӧ����A�ĵ��ʡ�������Һ�к�������ԭ�ӵĵ��Ӳ�ṹ��ͬ�������ӡ�
�����������2��1��ȫ��Ӧ���������ڳ�������Һ�塣��Һ����D�����ܾ��ҷ�Ӧ����A�ĵ��ʡ�������Һ�к�������ԭ�ӵĵ��Ӳ�ṹ��ͬ�������ӡ� ___________��
___________��