��Ŀ����
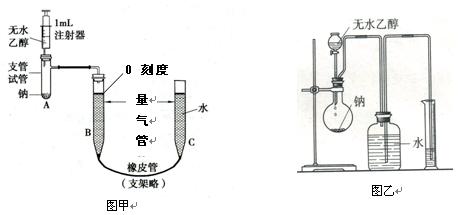
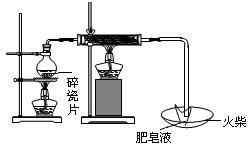
��10�֣�Ϊ�˲ⶨ�Ҵ��ķ��ӽṹ�����������ͼ����ʾ������ˮ�Ҵ����Ʒ�Ӧ���ⶨH2�������ʵ��װ�á��ش������й����⣺
��1���Ҵ����Ʒ�Ӧ����ʽΪ��__________________________________________________��
��2����ͼ������װ��ã�����C�ܣ�ʹB��C���ܼ����15~20cmˮλ�����ֹͣ���ò�����Ŀ���� ��
��3����֪��ˮ�Ҵ��ܶ�Ϊ0.789g/mL����ȡ0.5mL��ˮ�Ҵ�����0.25mL��ˮ�Ҵ���������A�У�ʹ֮���Ƴ�ַ�Ӧ����A������Ӧ�����Ƶ�����Ϊ ����С�������һλ���֣�
��4��ע����������0.5mL��ˮ�Ҵ������ƺõ��Ʒ���A�У��ڽ��Ҵ�����A��֮ǰӦ�ý��еIJ����� ��
��5��Ҳ�����������ͼ����ʾ�IJⶨ�Ҵ��ķ��ӽṹ��װ�á��Է���ʵ��װ�ü���ʵ��װ������ȣ��ŵ��� ��������㼴�ɣ���

��1���Ҵ����Ʒ�Ӧ����ʽΪ��__________________________________________________��
��2����ͼ������װ��ã�����C�ܣ�ʹB��C���ܼ����15~20cmˮλ�����ֹͣ���ò�����Ŀ���� ��
��3����֪��ˮ�Ҵ��ܶ�Ϊ0.789g/mL����ȡ0.5mL��ˮ�Ҵ�����0.25mL��ˮ�Ҵ���������A�У�ʹ֮���Ƴ�ַ�Ӧ����A������Ӧ�����Ƶ�����Ϊ ����С�������һλ���֣�
��4��ע����������0.5mL��ˮ�Ҵ������ƺõ��Ʒ���A�У��ڽ��Ҵ�����A��֮ǰӦ�ý��еIJ����� ��
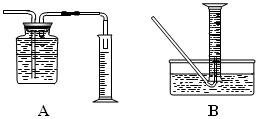
��5��Ҳ�����������ͼ����ʾ�IJⶨ�Ҵ��ķ��ӽṹ��װ�á��Է���ʵ��װ�ü���ʵ��װ������ȣ��ŵ��� ��������㼴�ɣ���
��1��2C2H5OH+2Na 2C2H5O Na +H2�� ��2�֣�
2C2H5O Na +H2�� ��2�֣�
��2������װ�������� ��2�֣�
��3��0.1 g ��2�֣���д��λ���÷�
��4��ʹB��ˮ���ڡ�0���̶�������λ�ã���1�֣�����C�ܸ߶ȣ�ʹB��C����Һ����ƽ��1�֣�
��5����ԼҩƷ���������Ҵ��Ļӷ������ʵ��ȷ�ԣ����������ȡ�Ҵ����ȷ�ȣ���ȡH2������㣬ȷ�� ��2�֣�ÿ���һ�����1�֣�
 2C2H5O Na +H2�� ��2�֣�
2C2H5O Na +H2�� ��2�֣���2������װ�������� ��2�֣�
��3��0.1 g ��2�֣���д��λ���÷�
��4��ʹB��ˮ���ڡ�0���̶�������λ�ã���1�֣�����C�ܸ߶ȣ�ʹB��C����Һ����ƽ��1�֣�
��5����ԼҩƷ���������Ҵ��Ļӷ������ʵ��ȷ�ԣ����������ȡ�Ҵ����ȷ�ȣ���ȡH2������㣬ȷ�� ��2�֣�ÿ���һ�����1�֣�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
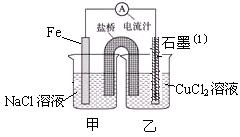
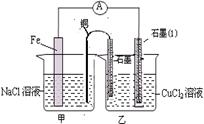
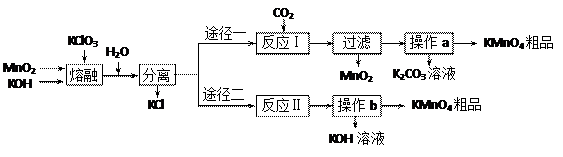 ��֪����3MnO2+KClO3+6KOH
��֪����3MnO2+KClO3+6KOH 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O �Ʒ�Ӧ�������������뻹ԭ��������ʵ���֮��Ϊ �� ���÷�Ӧ�п���ѭ�����õIJ����� �� ��
�Ʒ�Ӧ�������������뻹ԭ��������ʵ���֮��Ϊ �� ���÷�Ӧ�п���ѭ�����õIJ����� �� ��