��Ŀ����
[��ѧѡ�ޡ���2����ѧ�뼼��]��15�֣�
����ˮ��������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й�����ȡ������Ʒ��
�ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е��� ������ţ���
���û�������ȡ��ˮ ����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ�� �ܸĽ��أ��壮þ����ȡ����
��2�����á���������������Ũ��ˮ�д���Br2�����ô������ա������������Ҫ��Ӧ�ǣ�Br2+Na2CO3+H2O  NaBr + NaBrO3+6NaHCO3������1mol Br2ʱת�Ƶĵ���Ϊ mol��
NaBr + NaBrO3+6NaHCO3������1mol Br2ʱת�Ƶĵ���Ϊ mol��
��3����ˮ��þ��һ�ι�����������ͼ��
Ũ��ˮ����Ҫ�ɷ����£�
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ ����Ʒ2�Ļ�ѧʽΪ ��1LŨ��ˮ���ɵõ���Ʒ2������Ϊ g��
��4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ �����ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ ��
��1���ڢۢ�
��2��5/3
��3��Ca2�� +SO42��====CaSO4�� Mg(OH)2 69.6
��4��MgCl2 Mg + Cl2��
Mg + Cl2��
Mg + 2H2O Mg(OH)2 + H2��
Mg(OH)2 + H2��
���������������1���û�����ֻ�ܳ�ȥ��ˮ�е���������ܻ�ȡ��ˮ����������������е��Ǣڢۢܡ���2�����û��ϼ���������ƽ������ԭ����ʽ��3Br2+6Na2CO3+3H2O ==== 5NaBr + NaBrO3+ 6NaHCO3��������1mol Br2ʱת�Ƶĵ���Ϊ5/3mol����3������Ũ��ˮ�ijɷּ���������֪�������Ϊ�ø����ӳ�ȥŨ��ˮ�е����������Ҫ��Ӧ�����ӷ���ʽΪCa2�� +SO42��====CaSO4�������������ͼ֪����Ʒ2�Ļ�ѧʽΪMg(OH)2��1LŨ��ˮ��þ����28.8g�����ʵ���Ϊ1.2mol������þԪ���غ�֪�����ɵõ�Mg(OH)21.2mol������Ϊ69.6g����4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪMgCl2 Mg + Cl2�������ʱ����������ˮ���ڣ�������þ��ˮ������Ӧ����ɲ�Ʒþ�����ģ��йط�Ӧ�Ļ�ѧ����ʽΪMg + 2H2O
Mg + Cl2�������ʱ����������ˮ���ڣ�������þ��ˮ������Ӧ����ɲ�Ʒþ�����ģ��йط�Ӧ�Ļ�ѧ����ʽΪMg + 2H2O Mg(OH)2 + H2����
Mg(OH)2 + H2����
���㣺���黯ѧ�뼼������ˮ���ۺ����á�

����������ұ����Ӧ����ʱͨ�����ȷֽⷨ����
| A��NaCl | B��HgO | C��Cu2S | D��Al2O3 |
�����оٵ���Դ�����ڶ�����Դ����
| A�����ܡ����� | B�����ܡ�ˮ�� |
| C������������ | D��ú��ʯ�� |
��֪MgO��MgCl2���۵�ֱ�Ϊ2800�桢604�棬��MgO��MgCl2�������ں�ͨ���⣬���ɵõ�����þ����ˮ�к���MgCl2����ҵ�ϴӺ�ˮ����ȡþ����ȷ�ķ����� �� ��
A����ˮ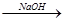 Mg(OH)2 Mg(OH)2 Mg Mg |
B����ˮ MgCl2��Һ MgCl2��Һ MgCl2���� MgCl2���� Mg Mg |
C����ˮ Mg(OH)2 Mg(OH)2 MgO MgO Mg Mg |
D����ˮ Mg(OH)2 Mg(OH)2 MgCl2��Һ MgCl2��Һ  MgCl2���� MgCl2���� Mg Mg |
������״̬�£�Na��KCl���ڿ��淴Ӧ��Na��KCl NaCl��K��ͨ�������¶ȣ������ý���Na����ȡK��
NaCl��K��ͨ�������¶ȣ������ý���Na����ȡK��
| ���� | K | Na | KCl | NaCl |
| �۵㣨�棩 | 63.6 | 97.8 | 770 | 801 |
| �е㣨�棩 | 774 | 883 | 1 500 | 1 413 |
�����ϱ����۵�ͷе㣬ȷ����Na��KCl��Ӧ��ȡK�ĺ����¶�Ϊ�� ��
A��770�� B��801��
C��850�� D��770�桫801��


