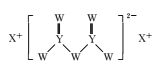
��Ŀ����
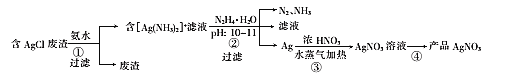
����Ŀ��һ�����ú�AgCl�ķ�����ȡAgNO3�Ĺ����������£�
(1)������ð�ˮ��ȡʱ���¶Ȳ��˳���35�棬��ԭ���� ___���ڰ�ˮŨ�ȡ���Һ�����Ⱥ��¶Ⱦ�һ��ʱ��Ϊ������Ľ�ȡ�ʻ��ɲ�ȡ�Ĵ�ʩ��____����ȡʱ������Ӧ�����ӷ���ʽΪ____��
(2)������з�����Ӧ�����ӷ���ʽΪ______��
(3)������У�����Ũ���ᷴӦ���ɵ����ʵ�����NO��NO2����������10.8g Agʱ����______molHNO3�����˷�Ӧ��
(4)����ܵIJ���Ϊ______��100���º�ɵò�ƷAgNO3��
(5)���������з�����������ԭ��Ӧ�IJ�����______(�á��١����ڡ����ۡ����ܡ����)��
���𰸡��¶ȹ�����ٰ��Ļӷ� �ʵ��ӳ���ȡʱ����߽������ٴν�ȡ AgCl+2NH3��H2O=[Ag(NH3)2]++Cl-+2H2O 4[Ag(NH3)2]++ N2H4��H2O=4Ag��+N2��+4NH4++4NH3��+H2O 0.15 ����Ũ������ȴ�ᾧ������(ϴ��) �ڢ�
��������
���Ȼ��������м��백ˮ��ȡ���õ�����[Ag(NH3)2]+����Һ���ʵ������¶ȿ��Լӿ췴Ӧ���ʣ��¶ȹ����ֻ���ٰ��Ļӷ�������[Ag(NH3)2]+����Һ�м���N2H4��H2O������pH��10��11���ù�����Ag+����ԭ��Ag��������N2��NH3�������˵õ�������������Ũ���ᣬ�õ���������Һ��֮������Ũ������ȴ�ᾧ������ϴ�ӵõ�AgNO3��
��1�������ӷ����¶ȹ�����ٰ��Ļӷ������ԭ�ϵ��˷ѣ��ڰ�ˮŨ�ȡ���Һ�����Ⱥ��¶Ⱦ�һ��ʱ��Ϊ������Ľ�ȡ�ʻ����ʵ��ӳ���ȡʱ����߽������ٴν�ȡ���������̿�֪��Ӧ��ΪAgCl�Ͱ�ˮ���������ӷ���ʽΪ��AgCl+2NH3��H2O=[Ag(NH3)2]++Cl-+2H2O��
��2����Ӧ����Ag+����ԭ��Ag��N2H4��H2O������ΪN2��ͬʱ�а������ɣ����ݵ����غ��Ԫ���غ�ɵ÷���ʽΪ��4[Ag(NH3)2]++ N2H4��H2O=4Ag��+N2��+4NH4++4NH3��+H2O��
��3��10.8g Ag�����ʵ���Ϊ0.1mol�������ɵ�n��AgNO3��=0.1mol�������ɵ�n��NO��= n��NO2��=x�����ݵ����غ��֪x+3x=0.1mol������x=0.025mol������ݵ�Ԫ���غ��֪���ĵ���������ʵ���Ϊ0.1mol+0.025mol+0.025mol=0.15mol��
��4������Һ�л�ȡ����һ����Ҫ����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�����
��5������ٵķ�Ӧû�л��ϼ۱仯������������ԭ���������AgԪ�ػ��ϼ۽��ͣ�NԪ�ػ��ϼ����ߣ�����������ԭ��Ӧ������������Ὣ��������������������ԭ��Ӧ�������Ϊ�����ᾧ���̣�û��Ԫ�ػ��ϼ۷����仯��������������ԭ��Ӧ��������������������ԭ��Ϊ�ڢۡ�

 ������ϵ�д�
������ϵ�д� �żӾ���ϵ�д�
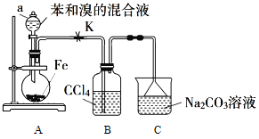
�żӾ���ϵ�д�����Ŀ���屽��һ����Ҫ����ԭ�ϣ�ʵ�����Ʊ��屽�ķ�Ӧװ����ͼ��ʾ���й��������£�
�� | �� | �屽 | |
��Է������� | 78 | 160 | 157 |
�ܶ�/gcm-3 | 0.88 | 3.10 | 1.50 |
�е�/�� | 80 | 59 | 156 |
ˮ���ܽ�� | �� | �� | �� |
�����кϳɲ���ش����⣺
��1������a������Ϊ___��
��2����Aװ���м���7.8mL��ˮ��������Һ̬���������м��ʵ��ʱװ��B�п��ܵ�����Ϊ___��װ��C��������___��
��3����Ӧ��ֺ�ȡAװ���з�ӦҺ���������в�������ᴿ��
�������м�������___�����Լ����ƣ���Ȼ��___����������ƣ���ȥδ��Ӧ����м��
����Һ������ˮ��l0%��NaOH��Һ��ˮϴ�ӡ�NaOH��Һϴ�ӵ�������___��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˡ�
��4�������Ϸ���������屽�л�����һ���������ʣ�Ҫ��һ���ᴿ�����������ڸ��ᴿ������û��ʹ�õ�����___(������ȷѡ��ǰ����ĸ)��
A. B.
B.![]() C. D.
C. D.
��5�����յõ���Ʒ5.5mL����ʵ��IJ�����___��