��Ŀ����
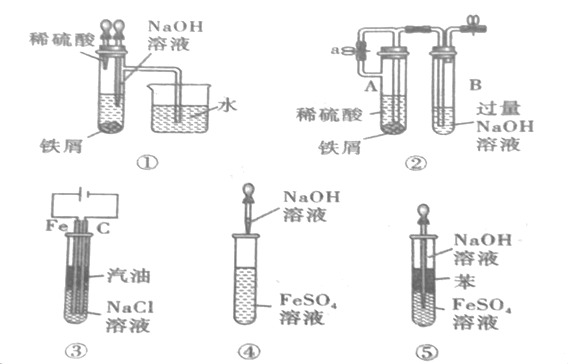
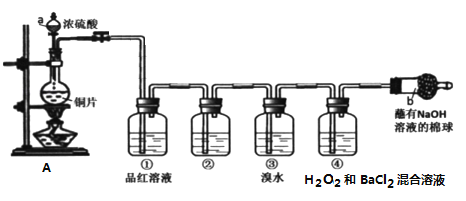
����Ŀ��ij�о���ѧϰС���������ͼװ����ȡ����֤SO2�����ʡ�
��ش�
(1)д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ_______________________________��
(2)������NaOH ��Һ��������_______________________________��д���÷�Ӧ���ӷ���ʽ_______________________________��
(3)Ϊ����֤SO2�����������ϴ��ƿ���п�ѡ����Լ���_____��
A.���Ը��������Һ B.��ɫ��̪��Һ C.����ʯ��ˮ D.��ˮ
(4)����˵����ȷ����_____��
A.ʵ�鿪ʼʱ��ֻ���Һ©��������������ʹҺ��˳������
B.����װ���м����Լ�(ҩƷ)���ٽ��������Լ��
C.ʵ�鿪ʼ��ϴ��ƿ�ٺ͢�����Һ����ɫ�����߾���֤��SO2����Ư����
D.ʵ�鿪ʼ��ϴ��ƿ���пɹ۲쵽��ɫ�����������������˵��SO2���л�ԭ��
(5)���������ŷŵ������У����γ����꣬���������������ú�pH_____(����С��)������Ϊ�������������;���ɲ�ȡ�Ĵ�ʩ��_____��
������ú��ȼ�ϣ� �ڰѹ����̴���ߣ� ��ȼ������ �������ữ�������м�ʯ�ң��ݿ����µ���Դ��
A.�ڢ� B.�ڢۢ� C.�٢ۢ� D.�٢ۢܢ�
���𰸡�Cu+2H2SO4 ![]() CuSO4+SO2��+H2O ���ն���Ķ�������ֹ��Ⱦ���� SO2+2OH-=SO32- +H2O C D ��С C
CuSO4+SO2��+H2O ���ն���Ķ�������ֹ��Ⱦ���� SO2+2OH-=SO32- +H2O C D ��С C
��������
Ũ�����Cu�ڼ��������·�Ӧ���ɶ���������Ʒ����Һ�����������Ҫ�������������������������Ҫͨ�����Ե�����֤�����������ˮ��Ӧ�����ἴ�ɣ��ܶ��������ܱ�����������������������ӣ���������Ӻͱ����ӷ�Ӧ���ɰ�ɫ���ᱵ���������������ж�����ֱ���ſգ������ü�Һ���ն�������β�����ݴ˷������
(1)װ��A��Ũ������ͭ��Ӧ���ɶ�������Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4 ![]() CuSO4+SO2��+H2O���ʴ�Ϊ��Cu+2H2SO4
CuSO4+SO2��+H2O���ʴ�Ϊ��Cu+2H2SO4 ![]() CuSO4+SO2��+H2O��
CuSO4+SO2��+H2O��
(2)���������ж�������ֱ���ŷţ���Ϊ����������������������Կ����ü�Һ���ն�������β����������NaOH��Һ�����������ն�������β������ֹ��Ⱦ��������Ӧ���ӷ���ʽΪSO2+2OH-=SO32- +H2O���ʴ�Ϊ�����ն�������β������ֹ��Ⱦ������SO2+2OH-=SO32- +H2O��
(3)Ϊ����֤SO2���������������ͨ�������������ˮ��Һ�����Լ��ɣ����������ˮ��Ӧ���������ᣬ���������������Ӷ�������Һ�����ԡ�A.���Ը��������Һ��ɫ�����ֶ�������Ļ�ԭ�ԣ���ѡ��B.��ɫ��̪��Һ�����ɫ����ѡ��C.����ʯ��ˮ�������ᷴӦ���ɱ�ɫ�������ܹ�˵�������������ԣ�ѡ��D.��ˮ��ɫ�����ֶ�������Ļ�ԭ�ԣ���ѡ���ʴ�Ϊ��C��
(4)A��ʵ�鿪ʼʱ��ֻ���Һ©������������Һ©����ƿ�������������ѹ���ڷ�Һ©������ѹ��Һ�岻��˳�����£���A����B��Ӧ���ȼ���װ�������ԣ�Ȼ����װ���м����Լ�(ҩƷ)����B����C����������ʹƷ����Һ��ɫ���ֶ��������Ư���ԣ����ܱ���������ԭ����HBr����ɫ������ˮ��ɫ���ֶ�������ԭ�ԣ�����ʵ�鿪ʼ��ϴ��ƿ�ٺ͢���Һ����ɫ��ǰ������Ư���ԡ��������ֻ�ԭ�ԣ���C����D��������������������������������ӣ���������Ӻͱ����ӷ�Ӧ�������ᱵ����������ʵ�鿪ʼ��ϴ��ƿ���пɹ۲쵽��ɫ�����������������˵��SO2���л�ԭ�ԣ���D��ȷ���ʴ�Ϊ��D��
(3)���������ŷŵ������У����γ����꣬���������ױ������������ᣬ���ú������������ǿ��pH��С����ԭú�к�����Ԫ�أ�����ԭú��ȼ�ϣ����ܼ��ٶ���������ŷţ����Կ��Լ�������IJ�������ȷ���ڰѹ������̴���ߣ������α������ܼ�������IJ���������ȼ�������Լ��ٶ���������ŷţ����Լ�������IJ�������ȷ���������ữ�������м�ʯ�ҵȴ�ʩ�����ܼ���������γɣ����ݿ����µ������Դ���ܼ��ٶ���������ŷţ����Լ�������IJ�������ȷ����ѡC���ʴ�Ϊ����С��C��

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�����Ŀ����Ҫ��ش���������
��1���ҹ��涨������ˮ�����ŷŵ��������Ũ��Ϊ0.005 mg/L���������ӷ�ˮ�ɲ��û�ѧ���������Իش��������⣺
�������ӣ�Cd3(PO4)2�������ܽ�ƽ�ⳣ���ı���ʽKsp��_____________________��
����ij���ӷ�ˮ�м���Na2S����S2-Ũ�ȴﵽ7.9 �� 10-8mol/Lʱ��ˮ����Cd2+Ũ��Ϊ_____mol/L����֪��Ksp��CdS��=7.9 �� 10-27��Cd�����ԭ��������112������ʱ�Ƿ����ˮԴ����______����������������������
��2����п�̳�����Ҫ�ɷ�ΪZnO��������CuO��FeO��Ϊԭ�ϣ�������ȡ�Ȼ�п�ͽ���п����ȡ�Ȼ�п��Ҫ�������£�

�±��г�����ؽ������������������������pH (��ʼ������pH����������Ũ��Ϊ1.0 mol��L��1����)��
�������� | Fe3+ | Zn2+ | Fe2+ |
��ʼ������pH | 1. 1 | 5. 2 | 5. 8 |
������ȫ��pH | 3. 2 | 6. 4 | 8. 8 |
������H2O2��Һ��������________________��
������ͼ�У�����pHʱ��������Լ�X������________������ţ���
a��ZnO b��NaOH c��Zn2(OH)2CO3 d��ZnSO4
pHӦ������______________________��