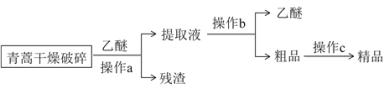
��Ŀ����
����Ŀ��������H�Ǻϳ�������Ѫ�ܼ���ҩ����м��壬��ͨ������;���ϳɣ�
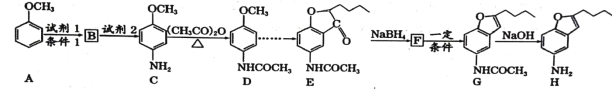
��֪:��![]() �������ױ�������
�������ױ�������
�ڼױ�����һ����ȡ����Ӧ��A���ơ�
�ش��������⣺
(1)д��������H�ķ���ʽ__________��C�к��������ŵ�����___________��
(2)д���йط�Ӧ���ͣ�B![]() C ___________��F
C ___________��F![]() G___________��
G___________��
(3)д��A![]() B�ķ�Ӧ����ʽ��___________________________ ��
B�ķ�Ӧ����ʽ��___________________________ ��
(4)д��ͬʱ������������D������ͬ���칹��Ľṹ��ʽ��____________
���ܷ���������Ӧ
���ܷ���ˮ�ⷴӦ��ˮ�����֮һ��FeCl3��Һ��Ӧ����ɫ
�ۺ˴Ź�������(1![]() ��ʾ��������4�ֲ�ͬ��ѧ��������
��ʾ��������4�ֲ�ͬ��ѧ��������
(5)�ϳ�;���У�Cת��ΪD��Ŀ����_____________________��
(6)���������ϳ�·�ߣ��Լױ���(CH3CO)2OΪԭ�ϣ����Լ���ѡ��������Ʊ�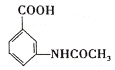 �ĺϳ�·�ߣ�_________________________
�ĺϳ�·�ߣ�_________________________
���𰸡�C12H15NO �Ѽ� ��ԭ��Ӧ ��ȥ��Ӧ ![]() +HNO3(Ũ)
+HNO3(Ũ) ![]()
 +H2O
+H2O 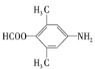 ��
�� ��
�� ������������ֹ�ϳɹ����б�����
������������ֹ�ϳɹ����б����� 
��������
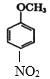
A��![]() ��A��ŨHNO3��ŨH2SO4���ȷ���ȡ����Ӧ����B��
��A��ŨHNO3��ŨH2SO4���ȷ���ȡ����Ӧ����B�� ��B��Fe��HCl������ԭ��Ӧ����C��
��B��Fe��HCl������ԭ��Ӧ����C��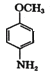 ��C��(CH3CO)2O����ȡ����Ӧ����D��
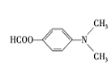
��C��(CH3CO)2O����ȡ����Ӧ����D��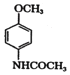 ��EΪ
��EΪ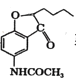 ��E��NaBH4������Ӧ����FΪ
��E��NaBH4������Ӧ����FΪ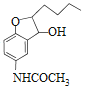 ��
��
(1)����H�Ľṹ��ʽ��֪�����ʽ��C12H15NO��C�ṹ��ʽ�� �����к��������ŵ�����Ϊ�Ѽ���
�����к��������ŵ�����Ϊ�Ѽ���
(2) BΪ ��CΪ
��CΪ ��B��Fe��HCl��Ӧ����C���÷�Ӧ����Ϊ��ԭ��Ӧ��
��B��Fe��HCl��Ӧ����C���÷�Ӧ����Ϊ��ԭ��Ӧ��
FΪ ��GΪ
��GΪ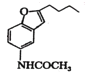 �����ݶ��߽ṹ�IJ�ͬ��֪��F
�����ݶ��߽ṹ�IJ�ͬ��֪��F![]() G����������ȥ��Ӧ��
G����������ȥ��Ӧ��
(3)A��![]() ��A��ŨHNO3��ŨH2SO4���ȷ���ȡ����Ӧ����B��
��A��ŨHNO3��ŨH2SO4���ȷ���ȡ����Ӧ����B�� ������A
������A![]() B�ķ�Ӧ����ʽΪ��
B�ķ�Ӧ����ʽΪ��![]() +HNO3(Ũ)
+HNO3(Ũ) ![]()
 +H2O��
+H2O��
(4)D�ṹ��ʽΪ�� ��D��ͬ���칹������������������ܷ���������Ӧ��˵������ȩ��-CHO��
��D��ͬ���칹������������������ܷ���������Ӧ��˵������ȩ��-CHO��
���ܷ���ˮ�ⷴӦ��ˮ�����֮һ��FeCl3��Һ��Ӧ����ɫ��˵������������ˮ����ﺬ�з��ǻ����������ʺ����ǻ��γɵ�������
�ۺ˴Ź�������(1![]() ��ʾ��������4�ֲ�ͬ��ѧ�������⣬����������ͬ���칹��ṹ��ʽΪ��
��ʾ��������4�ֲ�ͬ��ѧ�������⣬����������ͬ���칹��ṹ��ʽΪ��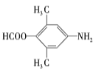 ��
�� ��
�� ��
��
(5)�ϳ�;���У�Cת��ΪDʱ��NH2������Ӧ����-NHCOCH3������ת��Ϊ������Ŀ���DZ�����������ֹ���ںϳɹ��̱�������
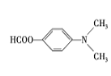
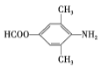
(6)���������ϳ�·�ߣ��Լױ���(CH3CO)2OΪԭ�ϣ�����Ʊ�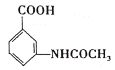 �ĺϳ�·�ߡ��ױ����ȱ�����KMnO4��Һ����Ϊ������
�ĺϳ�·�ߡ��ױ����ȱ�����KMnO4��Һ����Ϊ������![]() ����������Ũ���ᡢŨ�����ϼ��ȷ���ȡ����Ӧ�����������ױ�
����������Ũ���ᡢŨ�����ϼ��ȷ���ȡ����Ӧ�����������ױ�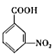 ��
�� ��Fe��HCl�����·�Ӧ����
��Fe��HCl�����·�Ӧ����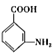 ��
�� ��(CH3CO)2�ڼ��������·���ȡ����Ӧ����
��(CH3CO)2�ڼ��������·���ȡ����Ӧ����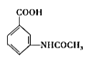 �����Լױ�ת��Ϊ
�����Լױ�ת��Ϊ �ĺϳ�·��Ϊ��
�ĺϳ�·��Ϊ�� 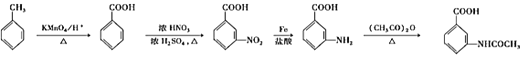

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ�������Ͻ�㷺Ӧ����ұ��ҵ������������ʱ�������������Ӽ��ȣ��ش��������⣺
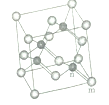
��1����̬Feԭ�Ӽ۲���ӵĵ����Ų�ͼΪ________����̬Siԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ________�Ρ�
��2������ʯ�����Ϊ![]() �������д�����������ʽΪ_____________.
�������д�����������ʽΪ_____________.
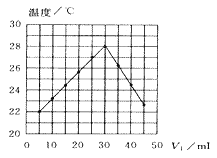
��3��![]() ���ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ________�����ӵ����幹��Ϊ________����±������ۡ��е����£�������仯���ɼ�ԭ��________________________________��
���ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ________�����ӵ����幹��Ϊ________����±������ۡ��е����£�������仯���ɼ�ԭ��________________________________��
|
|
|
| |
�۵�/K | 182.8 | 202.7 | 278.5 | 393.6 |
�е�/K | 177.4 | 330.1 | 408 | 460.6 |
��4��![]() �����Ҷ�����
�����Ҷ�����![]() ����дΪen���������·�Ӧ��
����дΪen���������·�Ӧ��![]()
![]() ��
��![]() ���������ӵ���λ��Ϊ________��
���������ӵ���λ��Ϊ________��![]() �е���λԭ��Ϊ________��
�е���λԭ��Ϊ________��
��5���ڹ������У�![]() �����壨ͼ
�����壨ͼ
|
|
ͼa | ͼb |
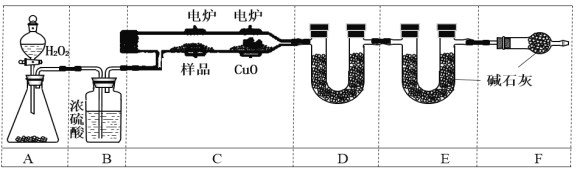
����Ŀ������װ����ʾ��ʵ���У����ܴﵽʵ��Ŀ����
|
|
|
|
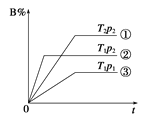
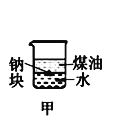
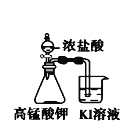
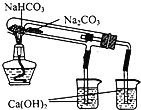
A����ʱ�俴��Fe(OH)2��ɫ���� | B��֤����(ú��)< ��(��) < ��(ˮ) | C��̽�������ԣ� KMnO4��Cl2��I2 | D���Ƚ�NaHCO3��Na2CO3�����ȶ��� |
A. A B. B C. C D. D