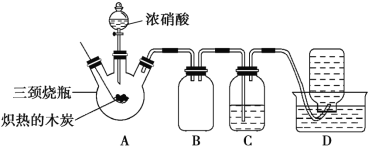
��Ŀ����
����Ŀ��NaClO2��һ����Ҫ��ɱ����������Ҳ������Ư��֯��ȣ���һ������������ͼ��ʾ����ش��������⣺
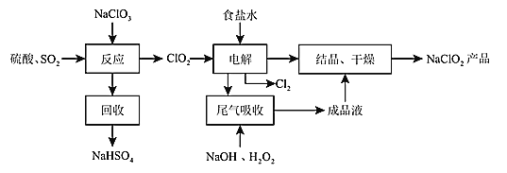
��1��NaClO2��ClԪ�صĻ��ϼ�Ϊ_____________��
��2��д������Ӧ������������ClO2�Ļ�ѧ����ʽ________________________��
��3����β�������������з����ķ�ӦΪ2NaOH + H2O2 + 2ClO2 = 2NaClO2 + O2�� + 2H2O �������������뻹ԭ�������ʵ���֮��Ϊ_________������3 mol ���ӷ���ת�ƣ�����__________L����״���£�O2���ɡ�
��4������Ч�Ⱥ���������������������������������������ָÿ�˺��������������������൱�ڶ��ٿ�Cl2��������������NaClO2����Ч�Ⱥ���Ϊ_____�����������λС������
���𰸡�+3 2NaClO3 + SO2 + H2SO4 = 2ClO2 + 2NaHSO4 2:1 33.6 1.57
��������
������ͼ��֪��NaClO3��SO2��H2SO4�ữ����������ClO2��NaHSO4����NaCl��Һ�м���ClO2�����е��ʱClO2�������õ�������ClO2-��Cl-������ʧ��������Cl2�����ú��й��������NaOH��Һ����δ�������ClO2���壬��Ӧ����NaClO2��O2��H2O��NaClO2��Һ�ᾧ������õ�NaClO2��Ʒ��
��1����NaClO2��NaΪ+1�ۣ�OΪ-2�ۣ������������ϼ۵Ĵ�����Ϊ0���ɵ�Cl�Ļ��ϼ�Ϊ+3�ۣ��ʴ�Ϊ��+3��
��2��NaClO3��SO2��H2SO4�ữ����������ClO2������NaClO2������������ԭ����ΪNaCl�����ݵ�ʧ�����غ��ԭ���غ㣬�˷�Ӧ�Ļ�ѧ����ʽΪ2NaClO3+SO2+H2SO4=2ClO2+2NaHSO4���ʴ�Ϊ��2NaClO3+SO2+H2SO4=2ClO2+2NaHSO4��
��3���ɷ�Ӧ����ʽ��֪����1mol��������Ӧ��ת��2mol���ӣ��������ͻ�ԭ�������ʵ���֮��Ϊ2:1��������3 mol ���ӷ���ת�ƣ���Ӧ�������������ʵ���Ϊ1.5mol�����Ϊ1.5mol��22.4L/mol=33.6L���ʴ�Ϊ��2:1��33.6��
��4��ÿ��NaClO2�����ʵ���n(NaClO2)=![]() =
=![]() mol����õ��ӵ����ʵ�����n(e)Ϊ
mol����õ��ӵ����ʵ�����n(e)Ϊ![]() mol��4��1 mol Cl2��õ��ӵ����ʵ�����2 mol�����ݵ���ת����Ŀ��ȣ���֪����������������ʵ���Ϊ
mol��4��1 mol Cl2��õ��ӵ����ʵ�����2 mol�����ݵ���ת����Ŀ��ȣ���֪����������������ʵ���Ϊ![]() mol��4��
mol��4��![]() =
=![]() mol��������������Ϊ
mol��������������Ϊ![]() mol��71 g/mol=1.57 g��NaClO2����Ч�Ⱥ���Ϊ1.57���ʴ�Ϊ��1.57��
mol��71 g/mol=1.57 g��NaClO2����Ч�Ⱥ���Ϊ1.57���ʴ�Ϊ��1.57��

 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�����Ŀ������������ζ����֮�ơ�������ʯ����Ҫ����SrSO4������CaCO3��MgO���ʣ������������ȵĹ������£�

��֪����������ˮ�е��ܽ�ȣ�
�¶ȣ��棩 | 0 | 10 | 20 | 30 | 40 | 60 | 80 | 90 | 100 |
�ܽ�ȣ�g/100mL�� | 0.91 | 1.25 | 1.77 | 2.64 | 3.95 | 8.42 | 20.2 | 44.5 | 91.2 |
��1��������������ʱSrSO4ֻ����ԭ��SrS����ѧ����ʽΪ____��
��2��������������������Һ������95������NaOH��Һ����pHΪ12��
��95��ʱˮ�����ӻ�KW��1.0��10��12��Ksp[Mg(OH)2]��1.2��10��10������Һ��c��Mg2������____��
����pH���������������ȵIJ��ʽ��ͣ������ԭ��____��
��3�������ȹ�������Ŀ����____��������������Ҫ�ɷ�Ϊ___��
��4���ӳ��ȹ��˺����Һ�еõ�Sr(OH)2��Ʒ�IJ���Ϊ____�����ˡ�ϴ�ӡ����
��5������������������FeCl3��Һ����������������壬����ʱ����������Ϊ___���ѧʽ��������ʯī�缫�������Һ�������������������ѭ�����õ�������__��
����Ŀ��ͨ�����ǰѲ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ��ܣ����ܵĴ�С�����ڹ��㻯ѧ��Ӧ�ķ�Ӧ��(��H)����ѧ��Ӧ����H���ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJ�.
��ѧ�� | H��H | Cl��Cl | H��Cl |
����1 mol��ѧ��ʱ�ų������� | 436 kJ/mol | 243 kJ/mol | 431 kJ/mol |
�������Ȼ�ѧ����ʽ����ȷ���� (����)
A.1/2H2(g)��1/2Cl2(g)===HCl(g)��H����91.5 kJ/mol
B.H2(g)��Cl2(g)===2HCl(g)����H����183 kJ/mol
C.2HCl(g)===H2(g)��Cl2(g)����H����183 kJ/mol
D.1/2H2(g)��1/2Cl2(g)===HCl(g)��H����91.5 kJ/mol