��Ŀ����
����Ŀ��A��B���Ƿ����廯���1molA ˮ��õ� 1molB �� 1mol ���ᡣA��B �����ƽ������������������200����ȫȼ�ն�ֻ����CO2 �� H2O���� B ��������Ԫ�ص������ٷֺ���Ϊ 41.56%������������Ϊ 0.4156����A ����Һ����̼��������Һ��Ӧ����ʹ FeCl3 ��Һ��ɫ��
��1��A��B �ķ�����֮��Ϊ__________��
��2��1 �� B ������Ӧ����__________����ԭ�ӡ�
��3��A �ķ���ʽ��__________��
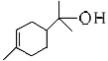
��4��B ������___________�ֽṹ���� B �ĺ˴Ź�������ͼ����4�����շ壬�� B �Ľṹ��ʽ������_____����дһ�ּ��ɣ���
���𰸡�42 4 C9H8O5 6 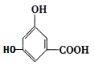 ��
��
��������
(1)���ݡ�1Ħ��Aˮ��õ�1Ħ��B��1Ħ�����ᡱ�ɱ�ʾΪ��A+H2O��B+CH3COOH�������������غ����⣻
(2)�����жϵĹ����ţ�������������ж���ԭ�ӵĸ�����
(3)����������Ӧ���ص��жϣ�
(4)����λ���칹����д�ṹ��ʽ��
(1)��1Ħ��Aˮ��õ�1Ħ��B��1Ħ�����ᡱ�ɱ�ʾΪ��A+H2O��B+CH3COOH�����������غ�֪��M(A)+18=M(B)+60����M(A)-M(B)=42���ʴ�Ϊ��42��
(2)��������Ϣ�Ƶ���A��B�������ص㣺�ٻ�����AΪ�������Һ����Ȼ��ͷ��ǻ����ڻ�����B����Է�������������158(200-42)����Ԫ�ص���������Ϊ41.56%��B�����г����Ȼ����2���ǻ�(����A�Ƴ�)������B����������4����ԭ��(1���Ȼ���2���ǻ�)����B������������4����ԭ�ӣ���ˣ�����ܵ���С��Է�������ΪM(B)=![]() =154��158������������⣬�ʴ�Ϊ��4��
=154��158������������⣬�ʴ�Ϊ��4��
(3)��(2)�ķ�����֪B�Ǻ���4����ԭ�ӵĶ��ǻ������ᣨC7H6O4����B���ǻ���CH3COOH��������������������A����ʽΪC9H8O5���ʴ�Ϊ��C9H8O5��
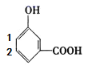
(4)��(2)�ķ�������֪��BӦΪ����������������-OH��-COOH�Ľṹ������������ȡ�������λ�ð�����һ��һԭ������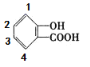 ��
�� ��6�ֽṹ��B �ĺ˴Ź�������ͼ����4�����շ壬����B�Ľṹ��ʽΪ��
��6�ֽṹ��B �ĺ˴Ź�������ͼ����4�����շ壬����B�Ľṹ��ʽΪ�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�����Ŀ����a��b��c��d��e�������壬�ֽ�������ʵ�飺
(1)a��b��� | ��������ɫ |
(2)c��d��� | �������� |
(3)c��e�ֱ�ͨ��������ˮ�� | ��ˮ�������ɫ����Һ�� |
(4)b��e�ֱ�ͨ���������� | ����������ɫ���� |
��a��b��c��d��e���ο����ǣ� ��
A. ![]() ��
��![]() ��HCl��
��HCl��![]() ��
��![]() B.
B. ![]() ��
��![]() ��
��![]() ��HCl��
��HCl��![]()
C. ![]() ��
��![]() ��
��![]() ��HCl��
��HCl��![]() D. HCl��
D. HCl��![]() ��
��![]() ��
��![]() ��
��![]()