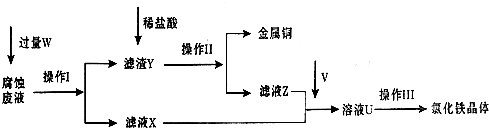
��Ŀ����
��12�֣�ijͬѧ��10mol• L-1 ��Ũ��������250mL 1mol• L-1 ��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
��1����Ҫ��ȡŨ����____________mL��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ����������⣬�������õ���������________________��___________��
��3��ʵ�����������������������з�����ȡ��4HCl��Ũ����MnO2 Cl2����MnCl2��2H2O��
Cl2����MnCl2��2H2O��
�Իش��������⣺
�÷�Ӧ��_____________��������������������_______________��
��������״���µ�Cl2 2.24L,��������HCl__________mol��
��1��25 ����2��250mL����ƿ ����ͷ�ιܣ� ��3�� MnO2 ��Cl2�� 0.2
����������1��������Һϡ��ʱ���ʵ��������䣬�ɼ������Ҫ��ȡŨ��������Ϊ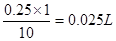 ��
��
��2���������ʵ���Ũ������Ҫ����Ͳ���ձ����������⣬�������õ���������250mL����ƿ �ͽ�ͷ�ιܡ�
��3���ɷ���ʽ��֪MnO2��MnԪ�صĻ��ϼ��ɣ�4�۽��͵���2�ۣ�����ԭ������������MnCl2�ǻ�ԭ���HCl��Cl�Ļ��ϼ��ɣ�1�����ߵ�0�ۣ�������������ԭ����Cl2�����������4molHClֻ��2mol����ԭ��������2mol�����Ե����á���״���µ�Cl2 ��2.24L�������ʵ�����0.1mol������ԭ���غ�֪��������HCl�����ʵ�����0.2mol��