��Ŀ����
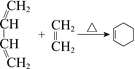
����Ŀ����ͼ��ʾ����Bˮ����װ��500 mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ������(��״��)�����Թ�A������Bˮ�۵�ˮ�С���ַ�Ӧ���Թ�A��������������Ϊ0.5a mL��
��1����A������B���з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________���÷�Ӧ���������뻹ԭ����������Ϊ________��ԭ���������NO2��NO�����ʵ���֮��Ϊ________��
��2��ͨ��������C������0.5a mL������Թ�A�г���ͨ��������A�п��ܹ۲쵽��������_______________________________________________________________________��
��3�����Թ�A�г�������ʱֹͣͨ��������Ȼ���Թ�ȡ��ˮ�ۣ�ˮ��B����Һ�����ʵ���Ũ��Ϊ________ mol��L1(����Һ�������Ϊ500 mL)��
���𰸡�3NO2+H2O===2HNO3+NO 1��2 3��1 ��ɫ�����Ϊ����ɫ���Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���� ![]()
��������
��1����A������Bˮ���з�����Ӧ3NO2+H2O=2HNO3+NO�����ݻ��ϼ۱仯�ж�����������ԭ����������֮�ȣ��������������С�������ò�������������������������������NO����������֮�ȵ������ʵ���֮�ȣ�
��2��ʣ��NO������ͨ���������ᷢ��4NO+3O2+2H2O=4HNO3���ݴ˷���ʵ������
��3�����ݵ�Ԫ���غ��֪n��HNO3��=n��NO2��+n��NO�����ٸ���c=n/V�������������Ũ�ȡ�
��1����A������Bˮ���У�����������ˮ��Ӧ���������һ���������壬��Ӧ�Ļ�ѧ����ʽΪ3NO2+H2O=2HNO3+NO���÷�Ӧ��,����������+4��NԪ�ر�������+5�۵����ᣬͬʱ����ԭ��+2�۵�һ����������3mol�����������뷴Ӧ��������������ԭ�Ķ�������Ϊ1mol������ԭ������������2mol������������뻹ԭ��������֮��Ϊ1:2��������NO2��NO�Ļ������(��״��)�Թ�A������Bˮ���У���ַ�Ӧ���Թ�A��������������Ϊ0.5a mL����NOΪymL��NO2ΪxmL����
3NO2+H2O=2HNO3+NO
3 1
x x/3
���y+x/3=0.5a������Ϊx+y=a����x=0.75a��y=0.25a����ͬ���������֮�������ʵ���֮�ȣ����ԭ���������NO2��NO�����ʵ���֮��Ϊ3��1��
��2��ʣ��NO������ͨ�������ᷢ��4NO+3O2+2H2O=4HNO3����ɫ�����Ϊ����ɫ���壬�Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壬��˱�����ȷ���ǣ���ɫ�����Ϊ����ɫ���Թ���Һ�治��������ȫ����������ͨ���������Թ���Һ���½�����������ɫ���壻
��3�����ݵ�Ԫ���غ��֪ˮ����������������ʵ���Ϊn��HNO3��=n��NO2��+n��NO��=a/22400 mol���������Ũ��Ϊc(HNO3)=a/22400 mol��0.5L=a/11200 mol/L��

����Ŀ��ʵ��������һδ֪Ũ�ȵ�ϡ����,ijͬѧ�ⶨ�����Ũ����ʵ�����н���ʵ�顣������������:
(1)����100![]() 0.10
0.10![]() ����Һ��
����Һ��
����Ҫ��������:�������������ܽ���(��ȴ��)��ϴ��(����ϴ��Һ��������ƿ) ��__________ ��__________ ��__________�������ƺõ���Һ�����Լ�ƿ��,���ϱ�ǩ��
�ڳ���__________![]() �������ƹ�������������:��ƽ(�����롢����)��__________��__________��
�������ƹ�������������:��ƽ(�����롢����)��__________��__________��
(2)ȡ20.00 ![]() �������������ƿ��,���μ�2-3�η�̪��ָʾ��,���Լ����Ƶ�
�������������ƿ��,���μ�2-3�η�̪��ָʾ��,���Լ����Ƶ�![]() ����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
ʵ���� |
| �ζ����ʱ, | ������������/ |
1 | 0.10 | 22.62 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.80 | 20.00 |
�� �ζ��ﵽ�յ�ı�־��__________��
�� ������������,�ɼ�����������Ũ��ԼΪ__________(������λ��Ч����)��
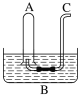
�� �ų���ʽ�ζ��������ķ���Ӧ������ͼ��ʾ�����е�__________,Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
�� ������ʵ����,���в���(����������ȷ)����ɲⶨ���ƫ�ߵ���__________(����ĸ���)��
A. �ζ��յ����ʱ����
B. ��ʽ�ζ���ʹ��ǰ,ˮϴ��δ�ô���������ϴ
C. ��ƿˮϴ��δ����
D. ������![]() �������
�������![]() ����
����
E. ��ʽ�ζ��ܼ��첿��������,�ζ�����ʧ