��Ŀ����
����Ŀ��ʵ��������һδ֪Ũ�ȵ�ϡ����,ijͬѧ�ⶨ�����Ũ����ʵ�����н���ʵ�顣������������:
(1)����100![]() 0.10
0.10![]() ����Һ��
����Һ��
����Ҫ��������:�������������ܽ���(��ȴ��)��ϴ��(����ϴ��Һ��������ƿ) ��__________ ��__________ ��__________�������ƺõ���Һ�����Լ�ƿ��,���ϱ�ǩ��
�ڳ���__________![]() �������ƹ�������������:��ƽ(�����롢����)��__________��__________��
�������ƹ�������������:��ƽ(�����롢����)��__________��__________��
(2)ȡ20.00 ![]() �������������ƿ��,���μ�2-3�η�̪��ָʾ��,���Լ����Ƶ�
�������������ƿ��,���μ�2-3�η�̪��ָʾ��,���Լ����Ƶ�![]() ����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
ʵ���� |
| �ζ����ʱ, | ������������/ |
1 | 0.10 | 22.62 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.80 | 20.00 |
�� �ζ��ﵽ�յ�ı�־��__________��
�� ������������,�ɼ�����������Ũ��ԼΪ__________(������λ��Ч����)��
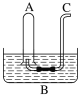
�� �ų���ʽ�ζ��������ķ���Ӧ������ͼ��ʾ�����е�__________,Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
�� ������ʵ����,���в���(����������ȷ)����ɲⶨ���ƫ�ߵ���__________(����ĸ���)��
A. �ζ��յ����ʱ����
B. ��ʽ�ζ���ʹ��ǰ,ˮϴ��δ�ô���������ϴ
C. ��ƿˮϴ��δ����
D. ������![]() �������
�������![]() ����
����
E. ��ʽ�ζ��ܼ��첿��������,�ζ�����ʧ
���𰸡���1�������� ��0.4 �ձ�(�������ҩ��
��2�������һ��NaOH��Һ���룬��Һ����ɫǡ�ñ�ɷۺ�ɫ ��0.11 mol/L ���� �� DE
��������
�������⣨1��������һ�����һ�����ʵ���Ũ�ȵ���Һ�IJ��������Ǽ������������ܽ���(��ȴ��)ת����ϴ��(����ϴ��Һ��������ƿ)�������� ҡ���� װƿ ����ǩ����n(NaOH)=" 0.100" L ��0.10 mol/L=0.01mol.m(NaOH)=0.01mol��40g/mol=0.4g.���Ҫ����0.4gNaOH. ���������У���ƽ(�����롢����)\�ձ���ҩ�ס���2��������������֪Ũ�ȵļ�ζ�δ֪Ũ�ȵ��ᣬ����ָʾ��������Һ�У����ζ��ﵽ�յ�ʱ�ῴ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ����ȷ���ζ��ﵽ���յ㡣��V(NaOH)= (22.62��22.72��22.80)ml��3=22.71ml.��ΪHClǡ�÷�Ӧʱ���ʵ����ı�Ϊ1:1.����c(HCl)=" (" 0.10 mol/L��22.71ml)��20.00ml=" 0.11" mol/L. �� ��ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����еı�������Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ����A���ζ��յ����ʱ���Ӷ���������ƫС������Һ�������ƫС�������������Һ��Ũ�Ⱦ�ƫ�͡�����B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ�������������Һ�����ʵ�����ƫ�٣��ζ�ʱ���ĵı���Һ�����ƫС�������������Һ��Ũ�Ⱦ�ƫ�͡�����C����ƿˮϴ��δ�������Ӱ��ʵ��ⶨ���������D���ζ�������,��������Һ������ƿ�⡣Ϊ�˽�������Һ���еζ�����Ҫ��μӱ���Һ������Һ���ƫ���ɴ˼����������Һ��Ũ�Ⱦ�ƫ�ߡ���ȷ��E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ����ʼ����ƫС����������ƫС�������ĵı���Һ�������ƫ�࣬�ɴ˼����������Һ��Ũ�Ⱦ�ƫ�ߡ���ȷ��

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�����Ŀ��ijС�����������װ�ý���ʵ�飬�ڡ�������Һ���������������������±���

ʵ�� | ���� | ���� |
�� | ��ʢ��Na2S��Һ�Ģ��г���ͨ��CO2������ | ���в�����ɫ��������Һ��pH���ͣ� ���в�����ɫ���ǣ��û�������ð���� |
�� | ��ʢ��NaHCO3��Һ�Ģ��г���ͨ��H2S���������� | ����ͬʵ��� |
���ϣ�CaS��ˮ��ȫˮ��
������ʵ��ó��Ľ�������ȷ����
A. ���а�ɫ������CaCO3
B. ������ҺpH���͵�ԭ���ǣ�H2S+Cu2+ == CuS��+2H+
C. ʵ������CO2���������ķ�Ӧ�ǣ�CO2+H2O+ S2== CO32+ H2S
D. ��ʵ���͢��ܱȽ�H2CO3��H2S���Ե�ǿ��