��Ŀ����
���Ṥҵ����Ӧ�����ۺϾ���Ч�����⡣
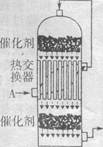
��1���ݲ��㣬�Ӵ�������������У�����Ӧ�ȶ�δ�����ã���ÿ����1 t 98%������������3.6��105 kJ����������ͨ�������жϣ�����ӦSO2(g)+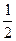 O2��g��
O2��g�� SO3����H="-98.3" kJ��mol-1�ų��������������������еõ�������ã���ÿ����1 t 98%������ֻ������ṩ�������������������ǧ����������H2SO4��Ħ������Ϊ98 g��mol-1��
SO3����H="-98.3" kJ��mol-1�ų��������������������еõ�������ã���ÿ����1 t 98%������ֻ������ṩ�������������������ǧ����������H2SO4��Ħ������Ϊ98 g��mol-1��
��2��CuFeS2�ǻ��������һ�ɷ֣�ȼ��ʱ��CuFeS2ת��ΪCuO��Fe2O3��SO2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�����᳧����¯�ų��Ŀ����к���Fe2O3��CuO��CuSO4����CuO��SO3�ڷ���¯�л��϶��ɣ�����������ͭ���������������¯�¶ȵIJ�ͬ���仯�����±�����
��֪CuSO4�ڵ���660 ��ʱ����ֽ⣬���Ҫ�����ϱ���CuSO4�������������¶����߶����͵�ԭ�� ��
��1���ݲ��㣬�Ӵ�������������У�����Ӧ�ȶ�δ�����ã���ÿ����1 t 98%������������3.6��105 kJ����������ͨ�������жϣ�����ӦSO2(g)+
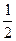 O2��g��
O2��g�� SO3����H="-98.3" kJ��mol-1�ų��������������������еõ�������ã���ÿ����1 t 98%������ֻ������ṩ�������������������ǧ����������H2SO4��Ħ������Ϊ98 g��mol-1��
SO3����H="-98.3" kJ��mol-1�ų��������������������еõ�������ã���ÿ����1 t 98%������ֻ������ṩ�������������������ǧ����������H2SO4��Ħ������Ϊ98 g��mol-1����2��CuFeS2�ǻ��������һ�ɷ֣�ȼ��ʱ��CuFeS2ת��ΪCuO��Fe2O3��SO2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�����᳧����¯�ų��Ŀ����к���Fe2O3��CuO��CuSO4����CuO��SO3�ڷ���¯�л��϶��ɣ�����������ͭ���������������¯�¶ȵIJ�ͬ���仯�����±�����
| ����¯�¶�/�� | 600 | 620 | 640 | 660 |
| ������CuSO4����������/% | 9.3 | 9.2 | 9.0 | 8.4 |
��1��6.23��105 kJ������
��2��4CuFeS2+13O2 4CuO+2Fe2O3+8SO2
4CuO+2Fe2O3+8SO2
��3��SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶ȵ����ߣ�ƽ�����淴Ӧ�����ƶ���SO3�����ʵ������٣�����CuSO4��������
��2��4CuFeS2+13O2
 4CuO+2Fe2O3+8SO2
4CuO+2Fe2O3+8SO2��3��SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶ȵ����ߣ�ƽ�����淴Ӧ�����ƶ���SO3�����ʵ������٣�����CuSO4��������
��1��SO2~SO3~H2SO4 �ų�������
98 g 98.3 kJ
1��106��98% g x
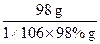 =
=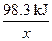
x=9.83��105 kJ
9.83��105 kJ-3.6��105 kJ=6.23��105 kJ
���Կ���������6.23��105 kJ������
��2��4CuFeS2+13O2 4CuO+2Fe2O3+8SO2
4CuO+2Fe2O3+8SO2
��3��SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶ȵ����ߣ�ƽ�����淴Ӧ�����ƶ���SO3�����ʵ������٣�����CuSO4��������
98 g 98.3 kJ
1��106��98% g x
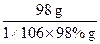 =
=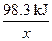
x=9.83��105 kJ
9.83��105 kJ-3.6��105 kJ=6.23��105 kJ
���Կ���������6.23��105 kJ������
��2��4CuFeS2+13O2
 4CuO+2Fe2O3+8SO2
4CuO+2Fe2O3+8SO2��3��SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ�����¶ȵ����ߣ�ƽ�����淴Ӧ�����ƶ���SO3�����ʵ������٣�����CuSO4��������

��ϰ��ϵ�д�
�����Ŀ
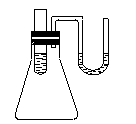
 ��1����Ʒ����Һ��ͨ����SO2�Ĺ����е�����Ϊ__________�����Ⱥ������Ϊ_______��
��1����Ʒ����Һ��ͨ����SO2�Ĺ����е�����Ϊ__________�����Ⱥ������Ϊ_______��