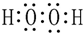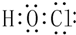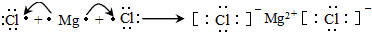��Ŀ����
4���������б仯��ϵ����ش��������⣺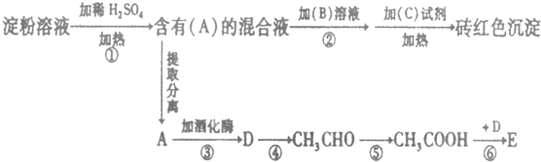
��1����д��A���ʵ����������ǣ�C�Լ������Ƶ�������ͭ����Һ����B��Һ������Ϊ����Һ���ɼ��ԣ�
��2����д��D���ʵ�һ����;��ȼ�ϣ�
��д��E���ʵĽṹ��ʽCH3COOCH2CH3��
д���آݲ���Ӧ�Ļ�ѧ����ʽ2CH3CHO+O2$\stackrel{��}{��}$2CH3COOH��
��3����ʯ���ѽ���IJ�����ϩ�ܺϳ����Ͼ���ϩ����д������ϩ�Ļ�ѧʽ��
 ��
��
���� �������и�����ת����ϵ���й������֪������������������ˮ��������ǣ�����AΪ�����ǣ��ڻ����Һ�мӹ���������������Һ����Һ���ɼ��ԣ��ټ����Ƶ�������ͭ����Һ�����ȳ���ש��ɫ����������BΪ����������Һ��CΪ���Ƶ�������ͭ����Һ���������ھƻ�ø�������µ�DΪCH3CH2OH��D����������Ӧ����ȩ����ȩ���������ᣬ�������Ҵ���Ӧ����EΪCH3COOCH2CH3���ݴ˴��⣮
��� �⣺�������и�����ת����ϵ���й������֪������������������ˮ��������ǣ�����AΪ�����ǣ��ڻ����Һ�мӹ���������������Һ����Һ���ɼ��ԣ��ټ����Ƶ�������ͭ����Һ�����ȳ���ש��ɫ����������BΪ����������Һ��CΪ���Ƶ�������ͭ����Һ���������ھƻ�ø�������µ�DΪCH3CH2OH��D����������Ӧ����ȩ����ȩ���������ᣬ�������Ҵ���Ӧ����EΪCH3COOCH2CH3��
��1����������ķ�����֪��AΪ�����ǣ�CΪ���Ƶ�������ͭ����Һ����B��Һ������Ϊ����Һ���ɼ��ԣ�
�ʴ�Ϊ�������ǣ����Ƶ�������ͭ����Һ������Һ���ɼ��ԣ�
��2��DΪCH3CH2OH����ҵ�ϳ��þƾ���ȼ�ϣ�EΪCH3COOCH2CH3����Ӧ�ݵĻ�ѧ����ʽΪ2CH3CHO+O2$\stackrel{��}{��}$2CH3COOH��
�ʴ�Ϊ����ȼ�ϣ�CH3COOCH2CH3��2CH3CHO+O2$\stackrel{��}{��}$2CH3COOH��
��3����ʯ���ѽ���IJ�����ϩ�ܺϳ����Ͼ���ϩ������ϩ�Ļ�ѧʽΪ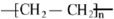 ��
��
�ʴ�Ϊ��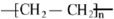 ��
��
���� ���⿼���л���ĺϳɣ���Ŀ�ѶȲ�����ע����ճ����л���Ĺ����ŵ����ʣ���ס��Ӧ�����������л��ﷴӦ�Ļ�ѧ����ʽҪ��д��

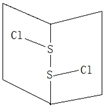 S2Cl2�dzȻ�ɫҺ�壮����й©�������Ϣ����ζ����ˮ���ɼ����ӷ�����������������Һ������ӽṹ��ͼ��ʾ�����й���S2Cl2��˵��������ǣ�������
S2Cl2�dzȻ�ɫҺ�壮����й©�������Ϣ����ζ����ˮ���ɼ����ӷ�����������������Һ������ӽṹ��ͼ��ʾ�����й���S2Cl2��˵��������ǣ�������| A�� | ��S2Br2�ṹ���ƣ��۷е�S2Br2��S2Cl2 | |
| B�� | �����мȺ��м��Լ��ֺ��зǼ��Լ� | |
| C�� | ��ˮ��Ӧ�Ļ�ѧ����ʽ����Ϊ2S2Cl2+2H2O=SO2��+3S��+4HCl | |
| D�� | Ϊ�Ǽ��Է��� |
��CH3Cl��CH2Cl2��CHCl3��CCl4��HCl��
| A�� | ֻ�Т٢� | B�� | �٢ڢۢܵĻ���� | C�� | ֻ�Тڢ� | D�� | �٢ڢۢܢݵĻ���� |
| A�� | A��B��C��D | B�� | A��C��B��D | C�� | A��C��D��B | D�� | D��B��A��C |
| A�� | ����ʯ��ˮ��������Ca��OH��2+2H+=Ca2++2H2O | |
| B�� | �������������ᷴӦH++OH-=H2O | |
| C�� | �Ȼ�����Һ��ϡ������Ba2++SO42-=BaSO4�� | |
| D�� | �������ṯ��Һ��ӦAl+Hg2+=Al3++Hg |
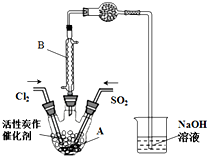 ijѧϰС�����ݷ�Ӧ��SO2��g��+Cl2��g��?SO2Cl2��g����H��0������Ʊ������ȣ�SO2Cl2����װ����ͼ���й���Ϣ�����ʾ��
ijѧϰС�����ݷ�Ӧ��SO2��g��+Cl2��g��?SO2Cl2��g����H��0������Ʊ������ȣ�SO2Cl2����װ����ͼ���й���Ϣ�����ʾ��| SO2Cl2 | Cl2 | SO2 | |
| �۵�/�� | -54.1 | -101 | -72.4 |
| �е�/�� | 69.1 | -34.6 | -10 |
| ���� | ��ˮ���� ����ˮ�� |
��2��B������������ʹ�ӷ��IJ���SO2Cl2����������
��3��Ϊ�˱��ڻ����ķ�������߷�Ӧ���ת���ʣ�Aװ�õķ�Ӧ�������ѡ��a��
a����ˮԡ b������ c��������69.1��
��4�����ͨ���Cl2��SO2����ˮ�����������Ͷ���������ܷ�����Ӧ�Ļ�ѧ����ʽΪSO2+Cl2+2H2O=H2SO4+2HCl��
��5��ʵ��ʱ��ͨ������Cl2��Aװ���еĿ������ߣ��ٻ���ͨ������SO2����������Ӧ����ַ�Ӧ����ͨ��Cl2ʹװ���е�SO2�����ձ��б����գ������������õ�SO2Cl2�м�ˮ�����ְ����������õõ���ɫ��ҺW��
�پ�����SO2Cl2��H2O��Ӧ���ڷ�������ԭ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽSO2Cl2+2H2O=H2SO4+2HCl��
����ɫ��ҺW�е������ӳ�������OH-�⣬�������������������ӣ�������ҺW�������������ӷ�����ȡ����W��Һ���Թ��У��������Ba��NO3��2��Һ���в�����ϡ����İ�ɫ����������˵����Һ�к���SO42-�����ˣ�����Һ�еμ�HNO3�ữ���ټ���AgNO3��Һ��������ɫ��������˵����Һ����Cl-��
�۷�Ӧ��ɺ���W��Һ���ձ��зֱ�μӹ�����BaCl2��Һ�������ְ�ɫ�������˳���������ϡ���ᣬ�����ˡ�ϴ�ӡ���������õ��Ĺ��������ֱ�ΪXg��Yg������SO2+Cl2?SO2Cl2��Ӧ�У�SO2��ת���ʣ��ú�X��Y�Ĵ���ʽ��ʾ����
| A�� | Cu | B�� | K2SO4 | C�� | SO2 | D�� | NaOH��Һ |
| A�� | 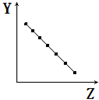 ��ʾ��������Ԫ�ص�ԭ�Ӱ뾶��ϡ��������⣩ | |
| B�� | 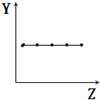 ��ʾ��A��Ԫ�ص����������� | |
| C�� | 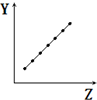 ��ʾ�ڶ�����Ԫ�صĵ縺�ԣ�ϡ��������⣩ | |
| D�� | 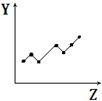 ��ʾ��������Ԫ�صĵ�һ�����ܣ�ϡ��������⣩ | |
| E�� | 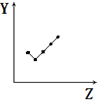 ��ʾ�ڢ�A��Ԫ���⻯��ķе㣮 |