��Ŀ����
����Ŀ��ˮ������Ҫ�Ľ������ϡ�ˮ�����ϵ���Ҫ�ɷ�ΪCaO��SiO2��������һ��������������þ�Ƚ����������ʵ���Ҳⶨˮ����Ʒ�иƺ����Ĺ�����ͼ��ʾ��

�ش��������⣺
��1���ڷֽ�ˮ����Ʒ�����У�������Ϊ�ܼ����Ȼ��Ϊ���ܼ���������뼸�����ᡣ���������Ŀ���ǽ�Fe2+����ΪFe3+ �������ӷ���ʽ________________________������ʹ��˫��ˮ�������ᣬ���ŵ���_________________________________________��
��2������B����Ҫ�ɷ���_____��_______��������B�м�����������������Һ��������_________���÷�Ӧ�����ӷ���ʽΪ____________________________________��
��3���Ӱ�ˮ�����������Һ��pHֵ�������pH��ֽ�ⶨ��Һ��pHֵ___________��
��4������Ƴ�����ϡH2SO4��������KMnO4����Һ�ζ���ͨ���ⶨ��������ɼ�ӻ�֪�Ƶĺ������ζ���ӦΪ��2![]() +6H++5H2C2O4=2Mn2++10CO2+8H2O��ʵ���г�ȡ0.800 gˮ����Ʒ���ζ�ʱ������0.100 mol��L-1��KMnO4��Һ36.00 mL�����ˮ����Ʒ�иƵ���������Ϊ______________��
+6H++5H2C2O4=2Mn2++10CO2+8H2O��ʵ���г�ȡ0.800 gˮ����Ʒ���ζ�ʱ������0.100 mol��L-1��KMnO4��Һ36.00 mL�����ˮ����Ʒ�иƵ���������Ϊ______________��
���𰸡�3Fe2+ + 4H+ + NO3��=3 Fe3+ + NO��+2H2O����Ϊˮ������ȾFe(OH)3Al(OH)3���������ܽ�Al(OH)3+OH-=AlO2��+2H2O��һС��pH��ֽ���ڱ�����(����Ƭ)�ϣ��ò�������������Һ������ֽ���в�����ֽ��ɫ�������ɫ���Ƚ���ȷ����Һ��pH(��պ���Ρ��ȡ���Ҳ����)45.0%
��������
ˮ�����ϵ���Ҫ�ɷ�ΪCaO��SiO2��������һ��������������þ�Ƚ���������������Ȼ�李���������ᣬ���ڶ������������Ӧ����õ��ij���AΪSiO2����Һ�к���Fe3+��Al3+��Mg2+�����ӣ����백ˮ����pH4-5��������Al(OH)3��Fe(OH)3���������ȵ�Ŀ���Ƿ�ֹ���ɽ�������Է��룬��Һ����Ҫ����Ca2+���������刺����ɲ���Ƴ��������������ø�����زⶨ������5Ca2+��5H2C2O4��2KMnO4�����ݸ�����ص����ɼ��㺬�����Դ˽�������
(1)�ڷֽ�ˮ����Ʒ�����У�������Ϊ�ܼ����Ȼ��Ϊ���ܼ���������뼸�����ᡣ���������Ŀ���ǽ�Fe2+����ΪFe3+ �������ӷ���ʽΪ3Fe2+ + 4H+ + NO3��=3 Fe3+ + NO��+2H2O������ʹ��˫��ˮ�������ᣬ˫��ˮ�Ļ�ԭ����Ϊˮ��������Ⱦ�������ʴ�Ϊ��3Fe2+ + 4H+ + NO3��=3 Fe3+ + NO��+2H2O������Ϊˮ������Ⱦ��
(2)������������������B����Ҫ�ɷ���Fe(OH)3��Al(OH)3��������B�м�����������������Һ����������������������Һ�ܽ⣬�����������ܣ�����Ϊ���������ܽ⣬��Ӧ�����ӷ���ʽΪAl(OH)3+OH-+=AlO2��+H2O���ʴ�Ϊ��Fe(OH)3��Al(OH)3�����������ܽ⣻Al(OH)3+OH-=AlO2��+2H2O��
(3)�Ӱ�ˮ�����������Һ��pHֵ����pH��ֽ�ⶨ��Һ��pHֵ�IJ���Ϊ����һС��pH��ֽ���ڱ�����(����Ƭ)�ϣ������д�����Һ�IJ�����������ֽ���в�����ֽ��ɫ�������ɫ���Ƚ���ȷ����Һ��pH�����Լ�Ϊպ���Ρ��ȡ������ʴ�Ϊ����һС��pH��ֽ���ڱ�����(����Ƭ)�ϣ��ò�������������Һ������ֽ���в�����ֽ��ɫ�������ɫ���Ƚ���ȷ����Һ��pH��
(4)��Ӧ�Ĺ�ϵʽΪ5Ca2+��5H2C2O4��2KMnO4��n(KMnO4)=0.0500mol/L��36.00mL=1.80mmol��n(Ca2+)=4.50mmol��ˮ���иƵ���������Ϊ![]() ��100%=45.0%���ʴ�Ϊ��45.0%��
��100%=45.0%���ʴ�Ϊ��45.0%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����1��һ���¶��£�Ksp[Mg3��PO4��2]��6.0��10��29��Ksp[Ca3��PO4��2]��6.0��10��26����Ũ�Ⱦ�Ϊ0.20mol��L��1��MgCl2��CaCl2�����Һ����μ���Na3PO4��������________�������ѧʽ�����������Һ����һ�ֽ��������ӳ�����ȫ��Ũ��С��10��5mol��L��1��ʱ����Һ�е���һ�ֽ��������ӵ����ʵ���Ũ��Ϊ________��
��2������ʯ����Ҫ�ɷ�BaCO3����Ca2����Mg2����Fe3�������ʣ���ʵ�������ö���ʯ�Ʊ�BaCl2��2H2O���������£�
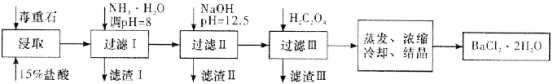
�ٶ���ʯ�������ȡǰ������ĥ��Ŀ����________��
�ڼ���NH3��H2O����pH��8�ɳ�ȥ________�������ӷ��ţ����������к�________���ѧʽ��������H2C2O4ʱӦ���������ԭ����________��
Ca2�� | Mg2�� | Fe3�� | |
��ʼ����ʱ��pH | 11.9 | 9.1 | 1.9 |
��ȫ����ʱ��pH | 13.9 | 11.1 | 3.7 |
��֪��Ksp��BaC2O4����1.6��10��7��Ksp��CaC2O4����2.3��10��9��
��3����֪25��ʱ��CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����100mL�������µ�CaSO4������Һ�м���400mL 0.01mol��L��1 Na2SO4��Һ������������ȷ����___������ĸ����
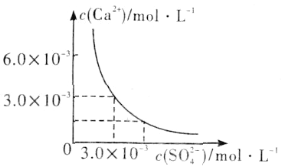
A����Һ������CaSO4������������Һ��c(SO42��)��ԭ���Ĵ�
B����Һ��������������Һ��c��Ca2������c(SO42��)����С
C����Һ������CaSO4��������Һ��c��Ca2������c(SO42��)����С
D����Һ��������������������Һ��c(SO42��)��ԭ���Ĵ�