��Ŀ����
5��2005��ŵ������ѧ�������ڷ����л���ϳ�ת�����������ܳ�����������ѧ�ң�����������•Ф������������•�������������•ʩ�ˣ���λ��ѧ�һ�ԭ����Ƕ��л���ѧϩ�����ֽⷴӦ���о��������ף���Ӧ�����ɱ�ʾΪ��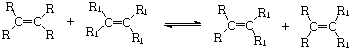
��֪ͬ��ͬѹ�£�C������������ܶ�Ϊ14��I�ķ���ʽΪC8H14O4���Ը�����ͼ��ϵ���ش����⣺
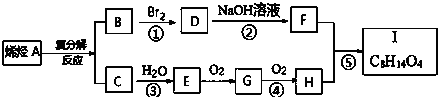
��1����֪C��ȼ����ΪQ kJ/mol��д��C��ȫȼ�յ��Ȼ�ѧ����ʽ��CH2=CH2��g��+3O2��g���T2CO2��g��+2H2O��l����H=-QkJ/mol��
��2��������D��E��G��F�У������ǻ�����EF��
��3����Ӧ�١��ڡ��ۡ��ܡ����У����ڼӳɷ�Ӧ���Ǣ٢ۣ�
��4��д��A�Ľṹ��ʽ��CH3CH=CH2��
��5��д��F+H��I��Ӧ�Ļ�ѧ����ʽ��
 ��
����6��д��G����������Ӧ�Ļ�ѧ����ʽ��
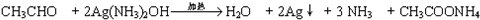 ��
��
���� ��֪ͬ��ͬѹ�£�C������������ܶ�Ϊ14����C����Է�������Ϊ28��C����ˮ�����ӳɷ�Ӧ����E����CΪCH2=CH2������EΪCH3CH2OH��������GΪCH3CHO��G�ٷ���������Ӧ��HΪCH3COOH��B���巢���ӳɷ�Ӧ��DΪ±������D�ڼ���������ˮ���FΪ����F��H����������Ӧ��I������I�ķ���ʽΪC8H14O4������֪BΪCH3CH=CHCH3��DΪCH3CHBrCHBrCH3��FΪCH3CHOHCHOHCH3��IΪCH3COOCH��CH3��CH��CH3��OOCCH3������������Ϣ��A����ϩ�����ֽⷴӦ��B��C������AΪCH3CH=CH2���ݴ˴��⣮
��� �⣺��֪ͬ��ͬѹ�£�C������������ܶ�Ϊ14����C����Է�������Ϊ28��C����ˮ�����ӳɷ�Ӧ����E����CΪCH2=CH2������EΪCH3CH2OH��������GΪCH3CHO��G�ٷ���������Ӧ��HΪCH3COOH��B���巢���ӳɷ�Ӧ��DΪ±������D�ڼ���������ˮ���FΪ����F��H����������Ӧ��I������I�ķ���ʽΪC8H14O4������֪BΪCH3CH=CHCH3��DΪCH3CHBrCHBrCH3��FΪCH3CHOHCHOHCH3��IΪCH3COOCH��CH3��CH��CH3��OOCCH3������������Ϣ��A����ϩ�����ֽⷴӦ��B��C������AΪCH3CH=CH2��
��1��CΪCH2=CH2����֪C��ȼ����ΪQ kJ/mol����C��ȫȼ�յ��Ȼ�ѧ����ʽΪCH2=CH2��g��+3O2��g���T2CO2��g��+2H2O��l����H=-QkJ/mol��
�ʴ�Ϊ��CH2=CH2��g��+3O2��g���T2CO2��g��+2H2O��l����H=-QkJ/mol��
��2����������ķ�����֪��������D��E��G��F�У�EΪCH3CH2OH��FΪCH3CHOHCHOHCH3�������ǻ���
�ʴ�Ϊ��EF��
��3����������ķ�����֪����Ӧ�١��ڡ��ۡ��ܡ����У����ڼӳɷ�Ӧ���Ǣ٢ۣ�
�ʴ�Ϊ���٢ۣ�
��4����������ķ�����֪��AΪCH3CH=CH2��
�ʴ�Ϊ��CH3CH=CH2��
��5��F+H��I��Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��GΪCH3CHO��G����������Ӧ�Ļ�ѧ����ʽΪ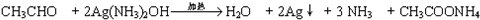 ��
��
�ʴ�Ϊ��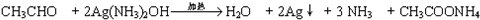 ��
��
���� ���⿼���л�����ƶ���ϳɣ���Ҫѧ���Է�Ӧ��Ϣ�������ã�����ʱע�����չ����ŵ�ת�����Ѷ��еȣ�

| A�� | ������Һ | B�� | �Ȼ�����Һ | C�� | �������� | D�� | ϡ���� |
| A�� | 18O2-�ṹʾ��ͼ��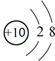 | |
| B�� | Na2O2�ĵ���ʽ��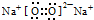 | |
| C�� | HCO3- ��ˮ�ⷽ��ʽHCO3-+H2O?H2CO3+OH- | |
| D�� | HI�ĵ��뷽��ʽ HI?H++I- |
| A�� | Fe3+��NH4+��SCN-��Cl- | B�� | Fe2+��H+��NO3-��SO42- | ||
| C�� | Fe2+��Fe3+��Na+��NO3- | D�� | Fe2+��NH4+��Cl-��OH- |
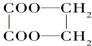 ʱ����������辭������Щ��Ӧ��������
ʱ����������辭������Щ��Ӧ����������ȡ���� �ڼӳ� ������ �ܻ�ԭ�� ����ȥ�� ��������
| A�� | �٢ڢۢܢ� | B�� | �ݢڢ٢ۢ� | C�� | �ޢۢ٢ڢ� | D�� | �٢ڢݢۢ� |
| A�� | ʯ����Ҫ��C H ����Ԫ�� | |
| B�� | ʯ������Ҫ�ɸ������� �������ͷ���������ɵĻ���� | |
| C�� | ʯ���й̶��ķе㣬�ʿɷ��� | |
| D�� | ʯ�ͷ���õ��������ǻ���� |
| A�� | ��״���£�22.4L���������еķ�����ΪNA | |
| B�� | 1mol����-CH3������������Ϊ9NA | |
| C�� | ��״���£�CH4��C2H4�Ļ������22.4L�������ķ�����ΪԼΪNA | |
| D�� | 26g C2H2�ͱ������Ļ��������������Cԭ����Ϊ2NA |
| A�� | �μ�ʯ����Һ�Ժ�ɫ����Һ��Fe3+��NH4+��Cl-��SCN- | |
| B�� | pHΪ1����Һ��Fe2+��K+��ClO-��Cl- | |
| C�� | c��H+��=10-12 mol•L-1����Һ��K+��Ba2+��Cl-��NO3- | |
| D�� | ���ڽ϶��H+��SO42-��NO3-����Һ��Al3+��CH3COO-��Cl- |
