��Ŀ����
��8�֣�����һ����Ҫ�Ļ���ԭ�ϣ����ĺϳɺ�Ӧ���ǵ�ǰ����Ҫ�о�����֮һ��
��ͳ�������ϳɰ���������صķ�ӦʽΪ��N2+3H2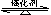 2NH3 �SH<0��
2NH3 �SH<0��
��1���÷�Ӧ��ƽ�ⳣ��K�ı���ʽΪ��K=______________�������¶ȣ�Kֵ______���������С�����䡱����
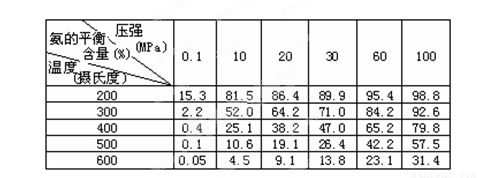
��2����ͬ�¶ȡ�ѹǿ�£��ϳɰ�ƽ����ϵ��NH3�����ʵ����������±���N2��H2����ʼ���ʵ���֮��Ϊ1��3���������������ݣ� �����¶Ⱥ�ѹǿ��ʱH2ת������ߣ�ʵ�ʹ�ҵ������ѡ�ø���������Ҫԭ���� ��
��8�֣���1�� ��2�֣��� ����2�֣���2��200�桢100MPa����2�֣�ѹǿ̫�ߣ��������豸Ҫ��Ҳ�ߣ�����ʵ�֣�2��,��
��2�֣��� ����2�֣���2��200�桢100MPa����2�֣�ѹǿ̫�ߣ��������豸Ҫ��Ҳ�ߣ�����ʵ�֣�2��,��
����������

����һ����Ҫ�Ļ���ԭ�ϣ����ĺϳ���Ӧ���ǵ�����Ҫ�о�����֮һ����ͬ�¶ȡ�ѹǿ�£��ϳɰ�ƽ����ϵ��NH3�����ʵ����������±���N2��H2��ʼ���ʵ���֮��Ϊ1��3����
|
�⺬��(%) �¶�(��) | 0.1 | 10 | 20 | 30 | 60 | 100 |
| 200 | 15.3 | 81.5 | 86.4 | 89.9 | 95.4 | 98.8 |
| 300 | 2.2 | 52.0 | 64.2 | 71.0 | 84.2 | 92.6 |
| 400 | 0.4 | 25.1 | 38.2 | 47.0 | 65.2 | 79.8 |
| 500 | 0.1 | 10.6 | 19.1 | 26.4 | 42.2 | 57.5 |
| 600 | 0.05 | 4.5 | 9.1 | 13.8 | 23.1 | 31.4 |
�ش������й����⣺
��1�����ñ��������ƶϵó��ϳɰ��ķ�Ӧ��__________��Ӧ������ȡ��������ȡ����������仯������
��2�����ݱ������ݣ���200���100MPaʱ��ƽ����ϵ��NH3�����ʵ���������ߣ���ʵ�ʹ�ҵ������ѡ�ø���������Ҫԭ����___________________________________��
��3��һ�������£������ܱ������н��еĺϳɰ���Ӧ��ƽ���������������ʱ����ͬʱѹ������������������¶ȴ���ƽ�����ԭƽ����ȣ��뽫�й��������ı仯����������±��У����������С����������Ҳ���ܼ�С������
| ��Ӧ���� | ƽ�ⳣ��K | ����������� | |
| �仯��� |
��4����1molH2��1molN2ͨ��һ���������ܱ������У���һ���¶Ⱥʹ��������£���Ӧ�ﵽƽ�⣬���NH3�����ʵ���Ϊ0.3mol����ʱ������0.5molH2��0.5molN2����Ӧ�ﵽ�µ�ƽ��ʱ��NH3�����ʵ���Ϊ_____________��ѡ��𰸱�ţ���
A��0.3mol B��0.15mol C����0.15mol D������0.15mol����0.3mol
 ��
�� ѹǿ(Mpa)
ѹǿ(Mpa) ����ƽ
����ƽ 2NH3(g) ��H����ͼI�Ǻϳɰ���Ӧ�������뷴Ӧ�������ͼ��δʹ�ô�������ͼD�Ǻϳɚݷ�Ӧ��2L�����С���ͬͶ������¡���������������ʱ��ijһ��Ӧ�����ĸı�Է�Ӧ��Ӱ��ͼ��
2NH3(g) ��H����ͼI�Ǻϳɰ���Ӧ�������뷴Ӧ�������ͼ��δʹ�ô�������ͼD�Ǻϳɚݷ�Ӧ��2L�����С���ͬͶ������¡���������������ʱ��ijһ��Ӧ�����ĸı�Է�Ӧ��Ӱ��ͼ��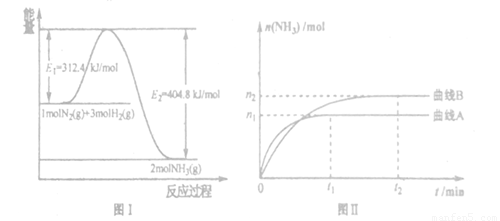
 mol/(L��min)
mol/(L��min) ��������¶��µ�ƽ�ⳣ��Ϊ_______����ͬһ�¶ȣ�ͬһ�����У�����ʼ���ʸ�Ϊamol N2 b molH2 c mol NH3 (a,b,c����Ϊ�㣩��ʹƽ�������и����ʵ�������ԭƽ����ͬ����a,b,c����Ĺ�ϵΪ_____________(�ú�a,b,c�ı���ʽ��ʾ��������ʹ��Ӧ����ʼʱ���淴Ӧ������У�c��ȡֵ��Χ��_______
��������¶��µ�ƽ�ⳣ��Ϊ_______����ͬһ�¶ȣ�ͬһ�����У�����ʼ���ʸ�Ϊamol N2 b molH2 c mol NH3 (a,b,c����Ϊ�㣩��ʹƽ�������и����ʵ�������ԭƽ����ͬ����a,b,c����Ĺ�ϵΪ_____________(�ú�a,b,c�ı���ʽ��ʾ��������ʹ��Ӧ����ʼʱ���淴Ӧ������У�c��ȡֵ��Χ��_______

 Cu + Cu2+����
Cu + Cu2+����