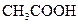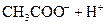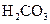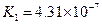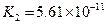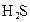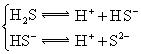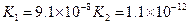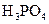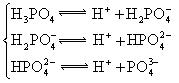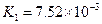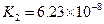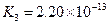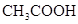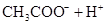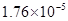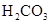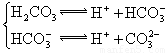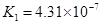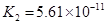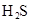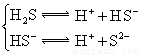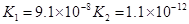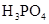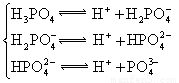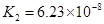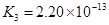��Ŀ����
| ��ѧʽ | HF | H2CO3 | HClO |
| ����ƽ�ⳣ�� ��K�� |
7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 |
3.0��10-8 |
��2��25��ʱ����20mL0.1mol?L-1������м���VmL0.1mol?L-1NaOH��Һ����û����Һ��pH�仯������ͼ��ʾ
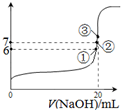 ������˵����ȷ����
������˵����ȷ����A��pH=3��HF��Һ��pH=11��NaF��Һ�У���ˮ�������c��H+�����
B���ٵ�ʱpH=6����ʱ��Һ�У�c��F-��-c��Na+��=9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c��F-��=c��Na+��
D���۵�ʱV=20mL����ʱ��Һ��c��F-����c��Na+��=0.1mol?L-1
��3����֪25��ʱ����HF��aq��+OH-��aq��=F-��aq��+H2O��l����H=-akJ?mol-1��
��H+��aq��+OH-��aq��=H2O��l����H=-bkJ?mol-1��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ
��4������������һֱ��Ϊ���ĺ�������ڣ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH-O-F��HFO������ʵ�����H2O��Ӧ�õ�HF�ͻ�����A����ÿ����1molHFת��
���Ȼ�������SO2Cl2����Ҫ�����Ȼ���������һ����ɫҺ�壬�۵�-54.1�棬�е�69.1�森�Ȼ��������ø���Ķ�������������ڻ���̿���������·�Ӧ��ȡ��
SO2��g��+Cl2��g��
 SO2Cl2��l����H=-97.3kJ?mol-1
SO2Cl2��l����H=-97.3kJ?mol-1��1����д�����³�ѹ�»�ѧƽ�ⳣ��K�ı���ʽ��K=
��2����������Ӧ����Ҫʹ��ѧƽ�ⳣ��K����ѧ��Ӧ����v��Ҳ���ɲ�ȡ�Ĵ�ʩ��
a�������¶� b������SO2Cl2
c�����ӷ�Ӧ��Ũ�� d����������������
��3��������������˵��������Ӧ�Ѵ�ƽ�����
a���ԣ�Cl2��=�ԣ�SO2�� b������������ѹǿ����ʱ����仯
c��c��Cl2����c��SO2��=1��1 d��������������ɫ����ʱ�����仯
��4��300��ʱ�����Ϊ1L���ܱ������г���16.20g SO2Cl2���ﵽƽ��ʱ�����к�SO2 7.616g�����������е�ƽ����ϵ�У��ټ���16.20g SO2Cl2�����ٴδ�ƽ��ʱ�������к�SO2��������Χ��
��2��A����������ˮ���룬�����������ӵ��δٽ�ˮ���룻
B�����ݵ���غ���㣻
C�����ݵ���غ���㣻
D�������ʵ�������������������ǡ�÷�Ӧ���ɷ����ƣ���Һ�ʼ��ԣ����ݵ���غ��жϣ�ע���������ʱ����ˮ������Ũ�ȱ�Ϊԭ����һ�룻
��3�����ø�˹���ɷ�����ע���������������ʣ�
��4������ԭ���غ�ȷ��A���ٸ��ݻ��ϼ۱仯����ת�Ƶ��ӣ�
II����1����ѧƽ�ⳣ����ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ���ݴ���д��
��2��ƽ�ⳣ��ֻ���¶�Ӱ�죬����ѧƽ�ⳣ��K����Ӧʹƽ��������Ӧ�ƶ����÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ����Ӧ�����¶ȣ���ѧ��Ӧ���ʽ��ͣ��ݴ˽��
��3���ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��䣬�ٷֺ������䣬�Լ��ɴ���������һЩ���������䣬�ݴ˽��ѡ���жϣ�
��4���ټ���16.20g SO2Cl2��ƽ�������ɶ�������ķ����ƶ���ƽ��ʱ������������������Ե�ЧΪ����ѹǿ��SO2Cl2ת���ʽ��ͣ�ƽ��ʱ�������������С��ԭƽ��ʱ��2����
��2��A�����������ˮ���룬�����ƴٽ�ˮ���룬����pH=3��HF��Һ��pH=11��NaF��Һ�У���ˮ������IJ���ȣ��ʴ���
B���ٵ�ʱpH=6����Һ�д��ڵ���غ㣬c��F-��+c��OH-��=c��Na+��+c��H+��������c��F-��-c��Na+��=c��H+��-c��OH-��=9.9��10-7mol/L������ȷ��
C���ڵ�ʱ����Һ�����ԣ�c��OH-��=c��H+������Һ�д��ڵ���غ㣬c��F-��+c��OH-��=c��Na+��+c��H+����
����c��F-��=c��Na+��������ȷ��
D���۵�ʱV=20mL����ʱ��Һ�������ڷ����ƣ���Һ�ʼ��ԣ����ݵ���غ�֪c��F-����c��Na+��������������ʱ��ˮ�������Ũ�ȱ�Ϊԭ����һ�룬����c��F-����c��Na+��=0.05mol/L���ʴ���
��ѡBC��
��3��ͨ������֪���������������ʣ�������ʽ��-�ڵ�HF��aq��?H+��aq��+F-��aq����H=-��a-b��KJ?mol-1���ʴ�Ϊ��HF��aq��?H+��aq��+F-��aq����H=-��a-b��KJ?mol-1��
��4������ԭ���غ�֪���÷�Ӧ����ʽΪ��HFO+H2O=HF+H2O2������Ԫ�ػ��ϼ�֪��ÿ����1molHFת�� 1mol���ӣ��ʴ�Ϊ��1��
II����1������ƽ�ⳣ����ʽ֪���÷�Ӧ��ƽ�ⳣ��K=
| 1 |
| c(SO2)��c(Cl2) |
�ʴ�Ϊ��
| 1 |
| c(SO2)��c(Cl2) |
��2��ƽ�ⳣ��ֻ���¶�Ӱ�죬����ѧƽ�ⳣ��K����Ӧʹƽ��������Ӧ�ƶ����÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ����Ӧ�����¶ȣ���ѧ��Ӧ���ʽ��ͣ��ʲ���ʵ��K�����ͬʱ��ѧ��Ӧ����v������ѡd��
��3��a���ԣ�Cl2��=�ԣ�SO2����û��ָ�����������ʣ����жϣ���a����
b���淴Ӧ���У���������ʵ�����С��ѹǿ��С������������ѹǿ����ʱ����仯��˵���ﵽƽ�⣬��b��ȷ��
c��ƽ��ʱ��������������Ũ������ʼŨ���йأ���ʼŨ�Ȳ�ͬ��ƽ��ʱ����Ũ�Ȳ�ͬ����������ʼŨ����ͬ�����ڶ��߰�1��1��Ӧ��������ʱ�̶��ߵ�Ũ�ȶ���ͬ����c��Cl2����c��SO2��=1��1����˵���ﵽƽ�⣬��c����
d��������������ɫ����ʱ�����仯��˵��������Ũ�Ȳ��ٱ仯��˵���ﵽƽ�⣬��d��ȷ��
�ʴ�Ϊ��bd��
��4���ټ���16.20g SO2Cl2��ƽ�������ɶ�������ķ����ƶ���ƽ��ʱ������������������Ե�ЧΪ����ѹǿ��SO2Cl2ת���ʽ��ͣ�ƽ��ʱ�������������С��ԭƽ��ʱ��2������ƽ��ʱ7.616g��m��SO2����15.232g��
�ʴ�Ϊ��7.616g��m��SO2����15.232g��

��.����ƽ�ⳣ������K��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
|
��ѧʽ |
HF |
H2CO3 |
HClO |
|
����ƽ�ⳣ�� ��K�� |
7.2��10-4 |
K1=4.4��10-7 K2=4.7��10-11 |
3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ����Na2CO3��Һ ��NaHCO3��Һ ��NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����______________��
��2��25��ʱ����20mL0.1mol��L��1������м���VmL0.1mol��L��1NaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_______��

A��pH��3��HF��Һ��pH��11��NaF��Һ�У���ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(F��)<c(Na+)��0.1mol��L��1
��3����֪25��ʱ����HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H��-akJ��mol��1��
��H+(aq)+OH��(aq)��H2O(l) ��H��-bkJ��mol��1��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ________________________��
��4������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO������ʵ�����H2O��Ӧ�õ�HF�ͻ�����A����ÿ����1molHFת��_______mol���ӡ�
��.�Ȼ�������SO2Cl2����Ҫ�����Ȼ���������һ����ɫҺ�壬�۵�C54.1�棬�е�69.1�档�Ȼ��������ø���Ķ�������������ڻ���̿���������·�Ӧ��ȡ��
SO2(g)��Cl2(g) SO2Cl2(l) ��H���C97.3kJ��mol��1
SO2Cl2(l) ��H���C97.3kJ��mol��1
��1����д�����³�ѹ�»�ѧƽ�ⳣ��K�ı���ʽ��K=_________________��
��2����������Ӧ����Ҫʹ��ѧƽ�ⳣ��K����ѧ��Ӧ����v��Ҳ���ɲ�ȡ�Ĵ�ʩ��_____��ѡ���ţ���
a�������¶� b������SO2Cl2
c�����ӷ�Ӧ��Ũ�� d����������������
��3��������������˵��������Ӧ�Ѵ�ƽ�����____________��ѡ���ţ���
a����(Cl 2)����(SO2) b������������ѹǿ����ʱ����仯
c��c(Cl 2) : c(SO2)��1:1 d��������������ɫ����ʱ�����仯
��4��300��ʱ�����Ϊ1L���ܱ������г���16.20g SO2Cl2���ﵽƽ��ʱ�����к�SO2 7.616g�����������е�ƽ����ϵ�У��ټ���16.20g SO2Cl2�����ٴδ�ƽ��ʱ�������к�SO2��������Χ��________________________��
�������ᣬ��һ���¶��´ﵽ����ƽ��ʱ��������Ũ�ȴ���һ�ֶ����Ĺ�ϵ���±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ��
|
�� |
���뷽��ʽ |
����ƽ�ⳣ��K |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�ش����и��ʣ�
��1��Kֻ���¶��йأ����¶�����ʱ��Kֵ________�����������С���������䡱����
��2�����¶���ͬʱ���������Kֵ��ͬ����ôKֵ�Ĵ�С�����Ե����ǿ���кι�ϵ?__________________��
��3������CH3COOH��H2CO3��HCO3-��H2S��HS-��H3PO4��H2PO4-��HPO42-���������ᣬ����������ǿ����_________����������________��
��4����Ԫ�����Ƿֲ�����ģ�ÿһ��������Ӧ�ĵ���ƽ�ⳣ��������ͬһ�ֶ�Ԫ�����K1��K2��K3֮������������ϵĹ��ɣ�����H3PO4�˹�����________________�������˹��ɵ�ԭ����_________________________��
 ��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH-
��֪0.10 mol��L-1
NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH-
��֪0.10 mol��L-1
NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
����pH��ֽ������Һ��pHֵ���������Cƽ����OH-�����ⶨ��ҺpHֵ�IJ�����______________��
�ڲ���Cƽ����NH3∙H2O���ķ��������_____________����������ƣ�
������¶��¸÷�Ӧ��ƽ�ⳣ��K.(д��������̣�����������2λ��Ч����)