��Ŀ����
��.����ƽ�ⳣ������K��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
|
��ѧʽ |
HF |
H2CO3 |
HClO |
|
����ƽ�ⳣ�� ��K�� |
7.2��10-4 |
K1=4.4��10-7 K2=4.7��10-11 |
3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ����Na2CO3��Һ ��NaHCO3��Һ ��NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����______________��
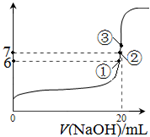
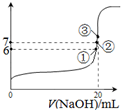
��2��25��ʱ����20mL0.1mol��L��1������м���VmL0.1mol��L��1NaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_______��

A��pH��3��HF��Һ��pH��11��NaF��Һ�У���ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(F��)<c(Na+)��0.1mol��L��1
��3����֪25��ʱ����HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H��-akJ��mol��1��
��H+(aq)+OH��(aq)��H2O(l) ��H��-bkJ��mol��1��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ________________________��
��4������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO������ʵ�����H2O��Ӧ�õ�HF�ͻ�����A����ÿ����1molHFת��_______mol���ӡ�
��.�Ȼ�������SO2Cl2����Ҫ�����Ȼ���������һ����ɫҺ�壬�۵�C54.1�棬�е�69.1�档�Ȼ��������ø���Ķ�������������ڻ���̿���������·�Ӧ��ȡ��
SO2(g)��Cl2(g) SO2Cl2(l) ��H���C97.3kJ��mol��1
SO2Cl2(l) ��H���C97.3kJ��mol��1
��1����д�����³�ѹ�»�ѧƽ�ⳣ��K�ı���ʽ��K=_________________��
��2����������Ӧ����Ҫʹ��ѧƽ�ⳣ��K����ѧ��Ӧ����v��Ҳ���ɲ�ȡ�Ĵ�ʩ��_____��ѡ���ţ���
a�������¶� b������SO2Cl2
c�����ӷ�Ӧ��Ũ�� d����������������
��3��������������˵��������Ӧ�Ѵ�ƽ�����____________��ѡ���ţ���
a����(Cl 2)����(SO2) b������������ѹǿ����ʱ����仯
c��c(Cl 2) : c(SO2)��1:1 d��������������ɫ����ʱ�����仯
��4��300��ʱ�����Ϊ1L���ܱ������г���16.20g SO2Cl2���ﵽƽ��ʱ�����к�SO2 7.616g�����������е�ƽ����ϵ�У��ټ���16.20g SO2Cl2�����ٴδ�ƽ��ʱ�������к�SO2��������Χ��________________________��
��. ��1���٢ܢڢۣ����>��>��>��,���������Ʊ�ʾҲ�ԣ���2�֣� ��2��BC��2�֣�
��3��HF(aq) 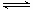 H+(aq)
+F��(aq) ��H��-(a-b)KJ��mol��1��2�֣���д����ſ�1�֣�
H+(aq)
+F��(aq) ��H��-(a-b)KJ��mol��1��2�֣���д����ſ�1�֣�
��4��1��2�֣�
��.��1��K��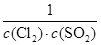 ��2�֣� 2��d��2�֣���3��bd��2�֣�
��2�֣� 2��d��2�֣���3��bd��2�֣�
��4��7.616g��m(SO2)��15.232g��2�֣�
��������
�����������.��1������Խ��Խˮ�⣬���볣��ԽС����ˮ��ʱ����Խǿ��̼��Ķ������볣����С�����Ԣټ�����ǿ������Ǣܣ��ٴ��Ǣڣ���С�Ǣۣ���2��A��HF��Һ�ж�ˮ�ĵ������������ã�NaF��Һ�ж�ˮ�ĵ�����ٽ����ã������ˮ�������c(H+)����ȣ�����B���ٵ�ʱpH��6�����ݵ���غ�C(Na+)+C(H+)=C(F-)+C(OH-)��c(F��)��c(Na+)= C(H+)- C(OH-)=10-6-10-8=9.9��10-7mol/L����ȷ��C���ڵ�ʱ��pH��7����Һ�����ԣ�C(H+)=C(OH-)�������Һ�е�c(F��)��c(Na+)����ȷ��D���۵�ʱV��20mL��ǡ����ȫ��Ӧ�õ�NaF��Һ��c(F��)<c(Na+)��0.05mol��L��1������ѡ��BC����3�����ݸ�˹���ɣ���-�ڿɵ�HF(aq) 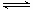 H+(aq)
+F��(aq) ��H��-(a-b)KJ��mol��1��4��H��O��F��F�ǽ�����ǿ�����õ��Ӷ�ƫ�������������-1��H�������+1��O��0�ۣ�HFO+H2O=HF+H2O2������1molHFת��1Ħ�����ӡ���.��2���÷�Ӧ�Ƿ��ȷ�Ӧ��ҪʹK����ֻ�ܽ����¶ȣ����·�Ӧ���ʽ��ͣ�����ѡd����3��a����(Cl 2)����(SO2)���κ������¶���ȣ�����b���������������������ϵ�����0������ѹǿ���ٸı�˵���������ʵ������ٱ仯���ﵽƽ�⣬��ȷ��c������Ũ��1��1������˵������Ũ�Ȳ��ٷ����仯������d����������ɫ����ɫ���䣬˵��Ũ�Ȳ��ٷ����仯��˵���ﵽƽ�⣬��ȷ����4��ƽ����ϵ�У��ټ���16.20g SO2Cl2��Ũ�ȱ�Ϊԭ����2������������ѹǿ��ƽ�������ƶ������Dz��ܵ����ı䣬���Ա�ԭ���Ĵ���2�����٣�7.616g��m(SO2)��15.232g
H+(aq)
+F��(aq) ��H��-(a-b)KJ��mol��1��4��H��O��F��F�ǽ�����ǿ�����õ��Ӷ�ƫ�������������-1��H�������+1��O��0�ۣ�HFO+H2O=HF+H2O2������1molHFת��1Ħ�����ӡ���.��2���÷�Ӧ�Ƿ��ȷ�Ӧ��ҪʹK����ֻ�ܽ����¶ȣ����·�Ӧ���ʽ��ͣ�����ѡd����3��a����(Cl 2)����(SO2)���κ������¶���ȣ�����b���������������������ϵ�����0������ѹǿ���ٸı�˵���������ʵ������ٱ仯���ﵽƽ�⣬��ȷ��c������Ũ��1��1������˵������Ũ�Ȳ��ٷ����仯������d����������ɫ����ɫ���䣬˵��Ũ�Ȳ��ٷ����仯��˵���ﵽƽ�⣬��ȷ����4��ƽ����ϵ�У��ټ���16.20g SO2Cl2��Ũ�ȱ�Ϊԭ����2������������ѹǿ��ƽ�������ƶ������Dz��ܵ����ı䣬���Ա�ԭ���Ĵ���2�����٣�7.616g��m(SO2)��15.232g
���㣺�����ε�ˮ�⡢����к͡��ȷ��̵���д����ѧ��Ӧԭ��

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�����ƽ�ⳣ������Ka��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
|
��ѧʽ |
HF |
H2CO3 |
HClO |
|
����ƽ�ⳣ�� ��Ka�� |
7.2��10-4 |
K1=4.4��10-7 K2=4.7��10-11 |
3.0��10-8 |
��1����֪25��ʱ����HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H����67.7kJ/mol��
��H+(aq)+OH��(aq)��H2O(l) ��H����57.3kJ/mol ��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ________________________��
��2����Ũ��Ϊ0.1 mol/LHF��Һ��ˮϡ��һ���������¶Ȳ��䣩�����и����������____��
A��c(H+) B��c(H+)��c(OH��) C�� D��
D��
��3��25��ʱ����20mL0.1mol/L������м���VmL0.1mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_____��

A��pH��3��HF��Һ��pH��11��NaF��Һ�У� ��ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(F��)< c(Na+)��0.1mol/L
��4�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ�� �� Na2CO3��Һ �� NaHCO3��Һ �� NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����______________��
��5��Na2CO3��Һ�Լ�������ΪCO32��ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤��֮
___________________________________________________________��
��6������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A��ÿ����1molHFת�� mol���ӡ�
 ������˵����ȷ����
������˵����ȷ���� SO2Cl2��l����H=-97.3kJ?mol-1
SO2Cl2��l����H=-97.3kJ?mol-1