��Ŀ����
��2011?���ϣ���ϩ��һ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ�����������ֻ�����Ʒ�ķ�Ӧ���£����ַ�Ӧ��������ȥ����

��ش��������⣺
��1��A�Ļ�ѧ������_
��2��B��A��Ӧ����C�Ļ�ѧ����ʽΪ
��3��D�Ľṹ��ʽΪ
 ��F�Ľṹ��ʽΪ
��F�Ľṹ��ʽΪ
 ��
��
��4��D��ͬ���칹��Ľṹ��ʽΪ

��ش��������⣺
��1��A�Ļ�ѧ������_
�Ҵ�
�Ҵ�
����2��B��A��Ӧ����C�Ļ�ѧ����ʽΪ
CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O
| Ũ���� |
| �� |
CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O
���÷�Ӧ������Ϊ| Ũ���� |
| �� |
������Ӧ
������Ӧ
����3��D�Ľṹ��ʽΪ




��4��D��ͬ���칹��Ľṹ��ʽΪ
CH3CHO
CH3CHO
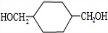
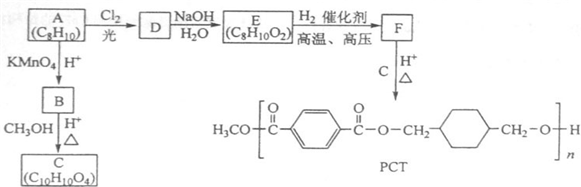
����������ϩ��ˮ�ӳ�����A��AΪ�Ҵ����Ҵ�����������B��BΪ���ᣬ�������Ҵ���Ӧ����C��CΪ������������ϩ��������Ӧ����D��C2H4O����DΪ�������� ����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ���
����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ��� ���ݴ˽��
���ݴ˽��
 ����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ���
����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ��� ���ݴ˽��
���ݴ˽������⣺��ϩ��ˮ�ӳ�����A��AΪ�Ҵ����Ҵ�����������B��BΪ���ᣬ�������Ҵ���Ӧ����C��CΪ������������ϩ��������Ӧ����D��C2H4O����DΪ�������� ����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ���
����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ��� ��
��
��1��������������֪��A���Ҵ����ʴ�Ϊ���Ҵ���
��2��B��A��Ӧ����C���������Ҵ���Ӧ����������������Ӧ����ʽΪ��
CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O���÷�Ӧ������Ϊ������Ӧ��
�ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��������Ӧ��
��3��������������֪��DΪ�������飬�ṹ��ʽΪ ��FΪ�������Ҷ������ṹ��ʽΪ
��FΪ�������Ҷ������ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��4�� ��ͬ���칹��Ľṹ��ʽΪCH3CHO���ʴ�Ϊ��CH3CHO��
��ͬ���칹��Ľṹ��ʽΪCH3CHO���ʴ�Ϊ��CH3CHO��
 ����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ���
����Ϊ����������Ҫ�����ᷢ��������Ӧ��1��2��Ӧ��D��E�ǿ����ӳɷ�Ӧ����EΪ�Ҷ������Ҷ��������ᷴӦ����F��C6H10O4����FΪ�������Ҷ��� ��
����1��������������֪��A���Ҵ����ʴ�Ϊ���Ҵ���
��2��B��A��Ӧ����C���������Ҵ���Ӧ����������������Ӧ����ʽΪ��
CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
��3��������������֪��DΪ�������飬�ṹ��ʽΪ
 ��FΪ�������Ҷ������ṹ��ʽΪ
��FΪ�������Ҷ������ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
����4��
 ��ͬ���칹��Ľṹ��ʽΪCH3CHO���ʴ�Ϊ��CH3CHO��
��ͬ���칹��Ľṹ��ʽΪCH3CHO���ʴ�Ϊ��CH3CHO�����������⿼���л��ƶϣ��漰ϩ��������ȩ������������Լ�ѧ�����������ȣ��Ƕ��л�������֪ʶ���ۺϿ��飬�ܽϺõĿ��鿼����˼ά������������ϩ��D�ķ���ʽ�����E�����ᷴӦ����F����������Cԭ����Ŀ���ж�D�Ľṹ�ǽ���Ĺؼ����Ѷ��еȣ��Ǹ߿��ȵ����ͣ�

��ϰ��ϵ�д�
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д�
�����Ŀ
 +2Cl2
+2Cl2 +2HCl
+2HCl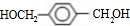 +3H2
+3H2