��Ŀ����
����Ŀ����һ������þ��ͭ��ɵĻ������뵽ϡ�����У�������ȫ�ܽ�(���跴Ӧ�л�ԭ����ֻ��NO)����Ӧ�����Һ�м���3 mol��L1NaOH��Һ��������ȫ��������ɳ�����������ԭ�Ͻ����������5.1 g��������������ȷ����
A. ������ȫ���ܽ�ʱ���μӷ�Ӧ����������ʵ���һ����0.4mol
B. �μӷ�Ӧ�Ľ�����������3.6g<m<9.6g
C. �����ɵij������ﵽ���ʱ������NaOH��Һ�����һ��ΪV=100mL
D. ������ȫ���ܽ�ʱ�ռ���NO���������ڱ�״����Ϊ2.24L
���𰸡�C
��������
��һ������þ��ͭ��ɵĻ������뵽ϡHNO3�У�������ȫ�ܽ⣨���跴Ӧ�л�ԭ����ֻ��NO����������Ӧ��3Mg+8HNO3 ��ϡ��=3Mg��NO3��2+2NO��+4H2O��3Cu+8HNO3 ��ϡ��=3Cu��NO3��2+2NO��+4H2O����Ӧ�����Һ�м��������3mol/LNaOH��Һ��������ȫ��������Ӧ��Mg��NO3��2+2NaOH=Mg��OH��2��+2NaNO3��Cu��NO3��2+2NaOH=Cu��OH��2��+2NaNO3������Ϊ������þ��������ͭ�����ɳ�����������ԭ�Ͻ����������5.1g����������þ��������ͭ����������������Ϊ5.1g�������������ʵ���Ϊ![]() =0.3mol�����ݵ���ת���غ㣬��þ��ͭ���ܵ����ʵ���Ϊ0.15mol��A�����ݷ���ʽ��֪�μӷ�Ӧ��n��Ӧ��HNO3��=
=0.3mol�����ݵ���ת���غ㣬��þ��ͭ���ܵ����ʵ���Ϊ0.15mol��A�����ݷ���ʽ��֪�μӷ�Ӧ��n��Ӧ��HNO3��=![]() n��������=0.15mol��
n��������=0.15mol��![]() =0.4mol����A��ȷ��B��þ��ͭ���ܵ����ʵ���Ϊ0.15mol���ٶ�ȫΪþ������Ϊ0.15mol��24g/mol=3.6g����ȫΪͭ������Ϊ0.15mol��64g/mol=9.6g�����Բμӷ�Ӧ�Ľ�������������m��Ϊ3.6g��m��9.6g����B��ȷ��C����������ʣ�࣬��μӷ�Ӧ�������Ƶ����ʵ���Ϊ0.3mol����Ҫ����������Һ���Ϊ��
=0.4mol����A��ȷ��B��þ��ͭ���ܵ����ʵ���Ϊ0.15mol���ٶ�ȫΪþ������Ϊ0.15mol��24g/mol=3.6g����ȫΪͭ������Ϊ0.15mol��64g/mol=9.6g�����Բμӷ�Ӧ�Ľ�������������m��Ϊ3.6g��m��9.6g����B��ȷ��C����������ʣ�࣬��μӷ�Ӧ�������Ƶ����ʵ���Ϊ0.3mol����Ҫ����������Һ���Ϊ��![]() =0.1L=100mL����������ʣ�࣬���ĵ�����������Һ�������100mL������V��100mL����C����D�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ
=0.1L=100mL����������ʣ�࣬���ĵ�����������Һ�������100mL������V��100mL����C����D�����ݵ���ת���غ��֪���ɵ�NO���ʵ���Ϊ![]() =0.1mol����״���£�����NO�����Ϊ0.1mol��22.4L/mol=2.24L����D��ȷ����ѡC��
=0.1mol����״���£�����NO�����Ϊ0.1mol��22.4L/mol=2.24L����D��ȷ����ѡC��

����Ŀ����þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO��ռ40%������������������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ��Դ�Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO47H2O�Ĺ����������£�
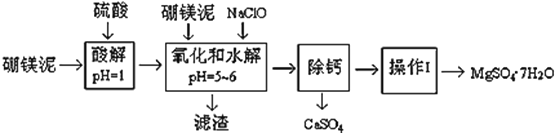
��1��ʵ������Ҫ1 mol/L������800 mL������ 98% ��Ũ���ᣨ��= 1.84 g/mL�������ƣ���ȡŨ������Ҫʹ����Ͳ�Ĺ��Ϊ__________����дѡ����ĸ��
A��10 mL B��20 mL C��50 mL D��100 mL
��2�������NaClO����Mn2+ ��Ӧ��Mn2+ + ClO + H2O = MnO2��+ 2H+ + Cl ���ڸò����л���һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ___________________��
��3����������Ҫ�ɷֳ�����Fe(OH)3��Al(OH)3�⣬������__________��___________��
��4���ڡ����ơ�ǰ���������Һ��Fe3+ �Ƿ������������鷽��___________________����д������������ͽ��ۣ�
��5����֪MgSO4��CaSO4 ���ܽ�ȣ���λΪ g/100 g ˮ�����±���
�¶ȣ��棩 | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
�����ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵����������______�����������ǽ���Һ��������Ũ������ȴ�ᾧ��______����õ���MgSO47H2O
��6��ʵ�����ṩ����þ�100 g���õ� MgSO47H2OΪ172.2 g ����MgSO47H2O �IJ���Ϊ___��
����Ŀ���������ӷ���ʽ����д�����۾���������( )
ѡ�� | ���ӷ���ʽ | ���� |
A | ��2 mol Cl2ͨ�뺬1 mol FeI2����Һ�У� 2Fe2����2I����2Cl2===2Fe3����4Cl����I2 | ��ȷ��Cl2�������ɽ�Fe2����I�������� |
B | Ba(HCO3)2��Һ��������NaOH��Һ��Ӧ�� Ba2����HCO3-��OH��===BaCO3����H2O | ��ȷ����ʽ����Ӧ�������κ�ˮ |
C | ����SO2ͨ��NaClO��Һ�У� SO2��H2O��ClO��===HClO��HSO3- | ��ȷ��˵�����ԣ�H2SO3ǿ��HClO |
D | 1 mol/L��NaAlO2��Һ��2.5 mol/L��HCl��Һ�������ϣ� 2AlO2-��5H��===Al3����Al(OH)3����H2O | ��ȷ����һ����Ӧ�͵ڶ�����Ӧ���ĵ�H�������ʵ���֮��Ϊ2��3 |
A. A B. B C. C D. D

