��Ŀ����
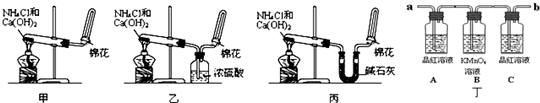
ij��ѧʵ��С��ͬѧ������ͼ1��ʾװ���Ʊ���������̽�����������ʣ�������������ȥ����

��ش�
��1��ʵ�����Ʊ������Ļ�ѧ����ʽΪ______�����ﰱ�����õĸ������______��
��2���ռ�����ʱ������ѡ�����Ľ�����______���a����b������������______��
��3�����۲쵽װ��B�е���ƿ�ڲ����˺�ɫ��Ȫ����˵���������е�������______��
��4��Ϊ��ֹ������Ⱦ����ͼ2��ʾװ�ã�ʢ�ŵ�Һ���Ϊˮ�����������ն��ఱ������______������ţ���
��5�������ڴ������Ҽ���ʱ�ᱻ�������������ǹ�ҵ������ĵ�һ����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ______��

��ش�
��1��ʵ�����Ʊ������Ļ�ѧ����ʽΪ______�����ﰱ�����õĸ������______��
��2���ռ�����ʱ������ѡ�����Ľ�����______���a����b������������______��
��3�����۲쵽װ��B�е���ƿ�ڲ����˺�ɫ��Ȫ����˵���������е�������______��
��4��Ϊ��ֹ������Ⱦ����ͼ2��ʾװ�ã�ʢ�ŵ�Һ���Ϊˮ�����������ն��ఱ������______������ţ���
��5�������ڴ������Ҽ���ʱ�ᱻ�������������ǹ�ҵ������ĵ�һ����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ______��
��1��ʵ������ȡ�����Ļ�ѧ����ʽ��2NH4Cl+Ca��OH��2
2NH3��+CaCl2+2H2O�������Ǽ������壬ѡ�ü�ʯ�Ҹ��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
2NH3��+CaCl2+2H2O����ʯ�ң�
��2�����ڰ����ܶȱȿ�����С��a�������������ſ�����
�ʴ�Ϊ��a����Ϊ�������ܶȱȿ����ᣬa�ڽ�������������
��3��������������ˮ�����������Ȫʵ�飬��ˮ������ܹ���������������ӣ�
�ʴ�Ϊ����������ˮ����ˮ��Ӧ���ɼ
��4�����ն��ڵİ���װ�ã������ܹ���ֹ������������������Тڢܢݣ�
�ʴ�Ϊ���ڢܢݣ�
��5�������������ķ�Ӧ����ʽ�ǣ�4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
| ||
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
��2�����ڰ����ܶȱȿ�����С��a�������������ſ�����
�ʴ�Ϊ��a����Ϊ�������ܶȱȿ����ᣬa�ڽ�������������
��3��������������ˮ�����������Ȫʵ�飬��ˮ������ܹ���������������ӣ�
�ʴ�Ϊ����������ˮ����ˮ��Ӧ���ɼ
��4�����ն��ڵİ���װ�ã������ܹ���ֹ������������������Тڢܢݣ�
�ʴ�Ϊ���ڢܢݣ�
��5�������������ķ�Ӧ����ʽ�ǣ�4NH3+5O2
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| �� |

��ϰ��ϵ�д�
�����Ŀ
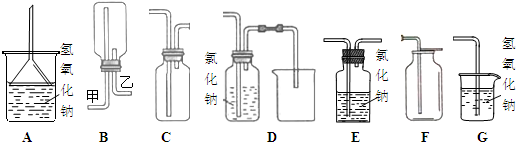
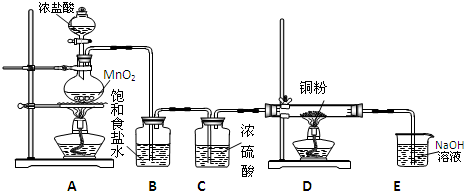
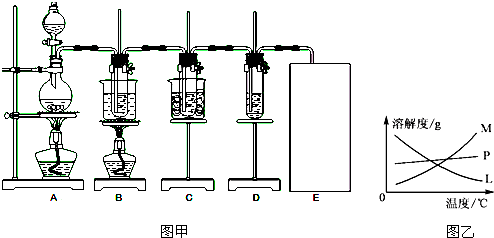



 �����������Ϣ�ش��������⣺
�����������Ϣ�ش��������⣺