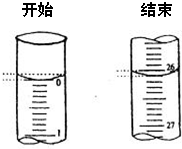
��Ŀ����
ijѧ����0.2000mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
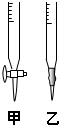
��������ˮϴ�Ӽ�ʽ�ζ��ܣ���ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壻
�۵���Һ������0����0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�������
��ش�
��1�����ϲ����д�����ǣ����ţ�______���ô�������ᵼ�²ⶨ������ƫ����ƫС������Ӱ�족��______
��2��������У�����ƿװҺǰ��������������ˮ���ⶨ������ƫ����ƫС������Ӱ�족��______
��3��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ��ⶨ������ƫ����ƫС������Ӱ�족��______
��4��������ʵ�����ݼ�¼��
ͨ������ɵã�������Ũ��Ϊ��______mol?L-1��������λ��Ч���֣�
��������ˮϴ�Ӽ�ʽ�ζ��ܣ���ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壻
�۵���Һ������0����0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�������
��ش�
��1�����ϲ����д�����ǣ����ţ�______���ô�������ᵼ�²ⶨ������ƫ����ƫС������Ӱ�족��______
��2��������У�����ƿװҺǰ��������������ˮ���ⶨ������ƫ����ƫС������Ӱ�족��______
��3��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ��ⶨ������ƫ����ƫС������Ӱ�족��______
��4��������ʵ�����ݼ�¼��
| �ζ����� | �������mL | NaOH��Һ���������ml�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 21.30 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
����1����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���NaOH��Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
��֪c������ƫ�ʴ�Ϊ���٣�ƫ��
��2������ƿװҺǰ��������������ˮ������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=
��֪c��������Ӱ�죬�ʴ�Ϊ����Ӱ�죻
��3���ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ����V������ƫС������c�����⣩=
��֪c������ƫС���ʴ�Ϊ��ƫС��
��4�����εζ����ĵ����Ϊ��21.30mL��16.30mL��16.22����ȥ��1�����ݣ�Ȼ�����2��3��ƽ������V��NaOH��=16.26mL��c�����⣩=
=
=0.1626mol?L-1���ʴ�Ϊ��0.1626��
| V(��)��c(��) |
| V(����) |
��2������ƿװҺǰ��������������ˮ������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=
| V(��)��c(��) |
| V(����) |
��3���ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ����V������ƫС������c�����⣩=
| V(��)��c(��) |
| V(����) |
��4�����εζ����ĵ����Ϊ��21.30mL��16.30mL��16.22����ȥ��1�����ݣ�Ȼ�����2��3��ƽ������V��NaOH��=16.26mL��c�����⣩=
| V(��)��c(��) |
| V(����) |
| 0.2000mol?L-1��16.26mL |
| 20.00mL |

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ