��Ŀ����
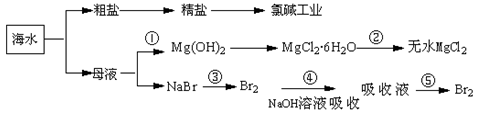
��12�֣��ۺ����ú�ˮ�����Ʊ�ʳ�Ρ��������þ��������ʣ�����������ͼ��ʾ��
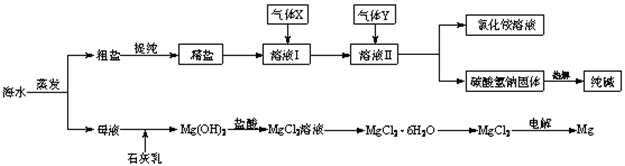
��1�������к��������ơ��Ȼ�þ���Ȼ��Ƶȿ��������ʣ�Ϊ��ȥ��Щ���ʶ��þ��Σ��������²��������ܽ⣻�ڼӹ�����BaCl2��Һ���ۼӹ�����NaOH��Һ���ܼӹ�����Na2CO3��Һ���������������������������������������������������������
��2����Һ���з�����Ӧ�Ļ�ѧ����ʽ�ǣ�����������������������������
��3��Mg(OH)2�����������Ca(OH)2����ѡ��___����___������Һ����ϴ���Գ�֮��
��4������������ˮ���Ȼ�þ����(MgCl2��6H2O)���ù���������þ����д���÷�Ӧ�Ļ�ѧ����ʽ________��������������������������__��������������
��5������ĸҺ��ͨ����������ȡ��ˮ�е��壬��Ӧ�����ӷ���ʽΪ��_____ _______��

��1�������к��������ơ��Ȼ�þ���Ȼ��Ƶȿ��������ʣ�Ϊ��ȥ��Щ���ʶ��þ��Σ��������²��������ܽ⣻�ڼӹ�����BaCl2��Һ���ۼӹ�����NaOH��Һ���ܼӹ�����Na2CO3��Һ���������������������������������������������������������
��2����Һ���з�����Ӧ�Ļ�ѧ����ʽ�ǣ�����������������������������
��3��Mg(OH)2�����������Ca(OH)2����ѡ��___����___������Һ����ϴ���Գ�֮��
��4������������ˮ���Ȼ�þ����(MgCl2��6H2O)���ù���������þ����д���÷�Ӧ�Ļ�ѧ����ʽ________��������������������������__��������������
��5������ĸҺ��ͨ����������ȡ��ˮ�е��壬��Ӧ�����ӷ���ʽΪ��_____ _______��
��1������ �����ᾧ��ֻд�����������֣�ֻд���ᾧ�������֣�
��2��NaCl + NH3 + CO2 + H2O��NaHCO3 + NH4Cl��2�֣������������ţ�
��3��MgCl2��д���Ȼ�þ��
��4��MgCl2��6H2O MgO+ 2HCl��+5H2O�� ��5��Cl2 + 2Br����2Cl��+ Br2
MgO+ 2HCl��+5H2O�� ��5��Cl2 + 2Br����2Cl��+ Br2
��2��NaCl + NH3 + CO2 + H2O��NaHCO3 + NH4Cl��2�֣������������ţ�
��3��MgCl2��д���Ȼ�þ��
��4��MgCl2��6H2O
 MgO+ 2HCl��+5H2O�� ��5��Cl2 + 2Br����2Cl��+ Br2
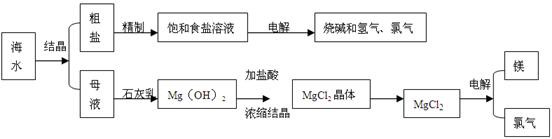
MgO+ 2HCl��+5H2O�� ��5��Cl2 + 2Br����2Cl��+ Br2��1��������ε��ᴿ����̼������Һ֮����Һ�к������ᱵ��������þ��̼�ᱵ�Լ�̼��Ƴ�������Ҫͨ�����˳�ȥ���ữ��ͨ�������ᾧ���õ��Ȼ��ơ�
��2������������̼�����ƺ��Ȼ�刺�֪����Ӧ�ķ���ʽΪNaCl + NH3 + CO2 + H2O��NaHCO3 + NH4Cl��
��3������������þ���ܽ��С���������Ƶģ�����Ҫ��ȥ������þ�е��������ƣ����Լ����Ȼ�þ����������þ���Ȼ��ƶ���ȥ��
��4���Ȼ�þ����Һ�д���ˮ��ƽ�⣬ˮ�������ȵģ�����ֱ�Ӽ��ȵò����Ȼ�þ�����ǵõ�����þ������ʽΪMgCl2��6H2O MgO+ 2HCl��+5H2O����
MgO+ 2HCl��+5H2O����
��5����Ԫ�صķǽ�����ǿ����Ԫ�صģ����������ܰ����������������嵥�ʣ�����ʽΪCl2 + 2Br����2Cl��+ Br2��
��2������������̼�����ƺ��Ȼ�刺�֪����Ӧ�ķ���ʽΪNaCl + NH3 + CO2 + H2O��NaHCO3 + NH4Cl��
��3������������þ���ܽ��С���������Ƶģ�����Ҫ��ȥ������þ�е��������ƣ����Լ����Ȼ�þ����������þ���Ȼ��ƶ���ȥ��
��4���Ȼ�þ����Һ�д���ˮ��ƽ�⣬ˮ�������ȵģ�����ֱ�Ӽ��ȵò����Ȼ�þ�����ǵõ�����þ������ʽΪMgCl2��6H2O
 MgO+ 2HCl��+5H2O����
MgO+ 2HCl��+5H2O������5����Ԫ�صķǽ�����ǿ����Ԫ�صģ����������ܰ����������������嵥�ʣ�����ʽΪCl2 + 2Br����2Cl��+ Br2��

��ϰ��ϵ�д�
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
�����Ŀ
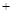 H2SO4��Ũ��
H2SO4��Ũ��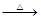 Li2SO4
Li2SO4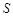 (Li2CO3)/g
(Li2CO3)/g

 2Na + Cl2��
2Na + Cl2�� 2Fe + 3CO2��
2Fe + 3CO2��
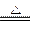 4Ag + O2��
4Ag + O2��