��Ŀ����
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
(1)��CO2�뽹̿��������CO��CO�����������ȡ�
��֪��Fe2O3(s)��3C(s)=2Fe(s)��3CO(g)����H1����489.0 kJ��mol��1
C(s)��CO2(g)=2CO(g)����H2����172.5 kJ��mol��1��
��CO��ԭFe2O3���Ȼ�ѧ����ʽΪ________________________________
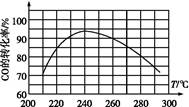
(2)ijʵ�齫CO2��H2����һ��������ܱ������У������ֲ�ͬ�����·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺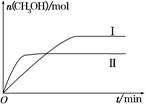
�ٸ÷�Ӧ��ƽ�ⳣ���ı���ʽΪK��________��
�����ߢ��Ӧ��ƽ�ⳣ����С��ϵΪK��________K��(����ڡ��������ڡ���С�ڡ�)��
������ͼa��b��c�����У�H2��ת�����ɸߵ��͵�˳����________(����ĸ)��
(3)�������������������£����������ѹ����ԭ����1/2����ԭƽ����ȣ������й�˵����ȷ����________(�����)��
a��������Ũ�ȼ�С
b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ�������
d������ƽ��ʱn(H2)/n(CH3OH)����
(1)Fe2O3(s)��3CO(g)=2Fe(s)��3CO2(g)����H����28.5 kJ��mol��1
(2)��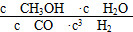 ���ڴ��ڡ���a��b��c
���ڴ��ڡ���a��b��c
(3)bc
����

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д���Ϊ��������ʵʩ�ġ��������䡱�����յ�վ�����Ϻ����ı���ú��ʯ��Ϊ������Դ�ṹ����Խ�����л�����Ⱦ�����ش�
(1)Ŀǰ�Ϻ��ֳ��о�����ʹ�õ�ȼ����Ҫ�ǹܵ�ú�����ֶ���������
��ʼʹ�ö�����Ȼ����Ϊ����ȼ�ϣ��ܵ�ú������Ҫ�ɷ���CO��H2������
���࣬��Ȼ������Ҫ�ɷ���CH4������ȼ�յĻ�ѧ����ʽΪ��
2CO��O2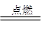 2CO2��2H2��O2
2CO2��2H2��O2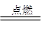 2H2O��CH4��2O2
2H2O��CH4��2O2 CO2��2H2O
CO2��2H2O
�������ϻ�ѧ����ʽ�жϣ�ȼ����ͬ����Ĺܵ�ú������Ȼ�������Ŀ�������ϴ�����������������ȼ�չܵ�ú����������������Ȼ������ߵĸĽ������������������(�����С��)���粻���Ľ����ܲ����IJ����������������������������������������
(2)�ܵ�ú���к��е����࣬�������⣬�����������顢���顢����ȣ����ǵ�ijЩ�������£�
| | ���� | ���� | ���� |
| �۵�/�� | ��183.3 | ��189.7 | ��138.4 |
| �е�/�� | ��88.6 | ��42.1 | ��0.5 |
�Ը�������ij���ؼ����ݽ��Ͷ����Ϻ��ļ�����ʱ�ܵ�ú�������С�����ҳʶ���״̬��ԭ����___________________________��
ͨ�����ǰѲ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ��,Ҳ���Թ��㻯ѧ��Ӧ�ķ�Ӧ��(��H),��ѧ��Ӧ�Ħ�H���ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJ
| ��ѧ�� | Si��O | Si��Cl | H��H | H��Cl | Si��Si | Si��C |
| ����/kJ��mol-1 | 460 | 360 | 436 | 431 | 176 | 347 |
��ش���������:
(1)�Ƚ������������ʵ��۵�ߵ�(�>����<��)��
SiC��������Si;SiCl4��������SiO2��
(2)��ͼ���������ĵġ�
 ����ʾ�辧���е�һ��ԭ��,����������Ķ����á�
����ʾ�辧���е�һ��ԭ��,����������Ķ����á� ����ʾ����֮���ڵĹ�ԭ�ӡ�
����ʾ����֮���ڵĹ�ԭ�ӡ�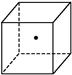
(3)��ҵ���øߴ����ͨ�����з�Ӧ��ȡ:SiCl4(g)+2H2(g)
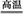 Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol��
Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol�� ����ϩ���ִ�ʯ�ͻ�����Ʒ������Ҫ�ĵ���֮һ���ڹ�ҵ�ϣ�����ϩ�����ұ���CO2
�������Ƶá��ܷ�Ӧԭ�����£�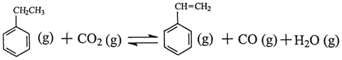 ��H
��H
�ش��������⣺
��1���ұ���CO2�����еķ�Ӧ�ɷ��������У�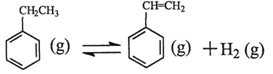 ��H1=��117.6kJ��mol��1
��H1=��117.6kJ��mol��1
H2 (g)��CO2 (g) CO (g)��H2O (g) ��H2=��41.2kJ��mol��1
CO (g)��H2O (g) ��H2=��41.2kJ��mol��1
���ұ���ȡ����ϩ��Ӧ��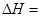 ��
��
��2�����¶�ΪT1ʱ���÷�Ӧ��ƽ�ⳣ��K=0.5mol/L����2L���ܱ������м����ұ���CO2����Ӧ��ijʱ�̲�û�����и���ֵ����ʵ�����Ϊ1.0mol��
�ٸ�ʱ�̻�ѧ��Ӧ ����ǡ����ǡ�������ƽ��״̬��
������������˵���ұ���CO2�ڸ������·�Ӧ�Ѵﵽƽ��״̬���� ������ȷ�𰸱�ţ���
a�������淴Ӧ���ʵı�ֵ�㶨 b��c(CO2)=c(CO)
c�����������ܶȲ��� d��CO2������������ֲ���
��������Ӧ��Ϊ��ѹ���������½��У��ﵽƽ��ʱ�����ұ������ʵ���Ũ�� ������ȷ�𰸱�ţ�
| A������0.5mol/L | B����0.5mol/L |
| C������0.5mol/L | D����ȷ�� |
 ����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú�
����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú� ��P�ı���ʽ��ʾ����
��P�ı���ʽ��ʾ������4��д���ɱ���ϩ��һ�������ºϳɾ۱���ϩ�Ļ�ѧ����ʽ ��
��������Ҫ�Ļ�����Ʒ֮һ��
(1)�ϳɰ��õ��������Լ���Ϊԭ���Ƶá��йػ�ѧ��Ӧ�������仯����ͼ��ʾ��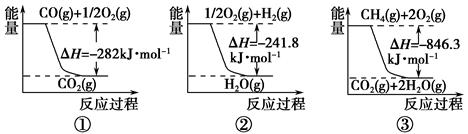
��Ӧ�٢ڢ�Ϊ________��Ӧ(����ȡ����ȡ�)��
CH4(g)��H2O(g)��Ӧ����CO(g)��H2(g)���Ȼ�ѧ����ʽΪ___________��
(2)�ð�����ȡ����[CO(NH2)2]�ķ�ӦΪ��2NH3(g)��CO2(g) CO(NH2)2(l)��H2O(g)��
CO(NH2)2(l)��H2O(g)��
��ij�¶��£����ݻ�Ϊ10 L���ܱ�������ͨ��2 mol NH3��1 mol CO2����Ӧ�ﵽƽ��ʱCO2��ת����Ϊ50%���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��______�����¶���ƽ�ⳣ��K�ļ�����Ϊ_____��
��Ϊ��һ�����CO2��ƽ��ת���ʣ����д�ʩ���ܴﵽĿ�ĵ���________��
| A�����NH3��Ũ�� | B������ѹǿ |
| C����ʱת�����ɵ����� | D��ʹ�ø���Ч�Ĵ��� |


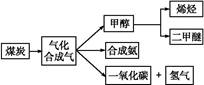
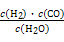 ,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:
,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:  CH3OH(g)
CH3OH(g)