��Ŀ����
��֪��������ͨ����������S8(б����)����ʽ���ڣ���������״̬ʱ������S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ����ͼ��ʾ��
��һ�������£�S8(s)��O2(g)������Ӧ����ת��ΪSO2(g)��SO3(g)����Ӧ���̺�������ϵ������ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol���������)��
��1��д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽ___________________________________��
��2��д��SO3�ֽ�����SO2��O2���Ȼ�ѧ����ʽ_______________________________________________________________��
��3����ѧ�Ϲ涨�����γ�1 mol��ѧ�����ջ�ų���������Ϊ�û�ѧ���ļ��ܣ���λkJ��mol������֪�������ļ���Ϊd kJ��mol��1���������ļ���Ϊe kJ��mol��1����S8������������ļ���Ϊ____________________________________��
��1��S8(s)��8O2(g)=8SO2(g)����H����8a kJ��mol��1
��2��2SO3(g)=2SO2(g)��O2(g)����H����2b kJ��mol��1
��3��(2d��a��e) kJ��mol��1
����

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1��
��2H2(g)��O2(g)=2H2O(l)����H����571.6 kJ��mol��1��
��C(s)��O2(g)=CO2(g)����H����393.5 kJ��mol��1��
(1)����д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų�������________(���������������)890.3 kJ��
(2)������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������
����ͼ�ܱ�ʾ�÷�Ӧ�����������仯����________(����ĸ)��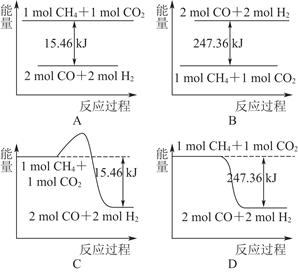
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ________��
(3)C(s)��H2(g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�������C(s)��2H2(g)=CH4(g)�ķ�Ӧ�Ȧ�H��________��
(4)Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���________(����ĸ)��
| A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų����� |
| B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2 |
| C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2) |
| D������̬̼�ϳ�ΪC60����C60��Ϊȼ�� |

 NH4++ NH2����ij�¶�ʱ�������ӻ�K=2��l0-30�����¶��£��ٽ�����NH4Cl�������Һ���У�K____________2��10-30�����������������=�������ڽ�����������Ͷ��Һ���У���ȫ��Ӧ��������Һ�и�����Ũ�ȴ�С��ϵΪ��_______
NH4++ NH2����ij�¶�ʱ�������ӻ�K=2��l0-30�����¶��£��ٽ�����NH4Cl�������Һ���У�K____________2��10-30�����������������=�������ڽ�����������Ͷ��Һ���У���ȫ��Ӧ��������Һ�и�����Ũ�ȴ�С��ϵΪ��_______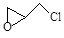 ������л���˴Ź��������� �� ���塣
������л���˴Ź��������� �� ���塣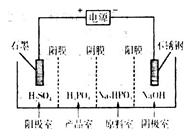
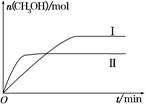
 CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺