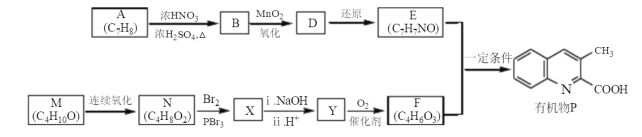
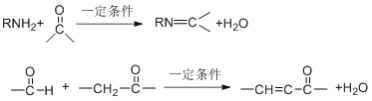
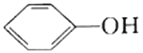
��Ŀ����
����Ŀ�����������(III)���K3[Fe(C204)3]��3H2OΪ��ɫ���壬������ˮ���������Ҵ���ͪ���л��ܼ���
I.���������(III)��ؾ�����Ʊ�
�ٽ�5g(NH4)2Fe(S04)2��6H2O��������20mLˮ�У�����5��6mol/LH2SO4�ữ�������ܽ⣬�����¼���25m���ͺ�H2C2O4��Һ�����ȣ����ã�����ɫ��Fe C2O4������ȫ�����Ժ���ȥ�ϲ���Һ��������ϴ�ӳ���2--3�Ρ�
��������м���10mL���Ͳ������Һ��ˮԡ������40�棬�õιܻ����μ�12mL5%H2O2���ӱ߽��貢ά����40�����ң���Һ�����ɫ������ɫ�ij������ɡ�
�ۼ�����ж�ʱ����ٷ�����������8mL����H2C2O4��Һ(�ȼ�5mL���������μ�3mL)��ʱ��ɫ�����ܽ⣬��Ϊ��ɫ����Һ��
������Һ�л�������10mL95%���Ҵ�����ʱ�����Һ���ǿ���ʹ����壬���ð�����ȴ���ᾧ��ȫ���ˣ�������ϴ����ϴ�Ӿ������γ�ɣ����������������ʡ�
��֪�Ƹ��������漰����Ҫ��Ӧ����ʽ����:
��6FeC2O4+3H2O2+6K2C2O4=4K3[Fe(C2O4)3]+2Fe(OH)3
�����2Fe(OH)3+3H2C2O4+3K2C2O4=2K3[Fe(C2O4)3]+6H2O
��ش����и���:
(1)��������������÷�Χ____________��
(2)����ۼ�����е�Ŀ����___________��
(3)��������Ҵ�Ҫ���������ԭ����_________��
(4)�������������ʺ���Ϊ����ϴ�Ӽ�����_______(����)��
A.��ˮ B.��ͪ C.95%���Ҵ� D.��ˮ�Ҵ�
(5)��ͼװ�ã�����һϵ�в�����ɾ���ij��˺�ϴ�ӡ���ѡ����ʵı�ţ�����ȷ��˳������(ϴ������ֻ��Ҫ����һ��):�������á�a��____��b��d��c���رճ����á�
a.ת�ƹ������� b.�ػ���A c.������A d.ȷ�ϳ�� e.��ϴ�Ӽ�ϴ��
II.���ȵIJⶨ
��ȡ1.000g��Ʒ�����Ƴ�250mL��Һ����ȡ25.00mL��Һ���ữ���ñ궨Ũ��Ϊ0.0100 mol/L �ĸ�������ܱ��ζ����յ㣬����ƽ��ʵ��ƽ�����ĸ�������ܱ�24.00 mL��
(6)�ζ��漰��Ӧ�����ӷ���ʽ:____________��
(7)�����Ʒ�Ĵ���______(�������ٷ�����ʾ)��(K3[Fe(C204)3]��3H2O����Է�������Ϊ491)
���𰸡����÷��뾧������ϴ��׳����������ײ��ij�����ȥ������˫��ˮ����߱���H2C2O4��Һ�������ʱ�������������쵼�¾�����СCbdce16H++2MnO4-+5C2O42-=2Mn2++10CO2��+8H2O98.20%
��������
����һʵ���Ʊ�Ϊ���壬����ѧ���Բ����ķ������ۡ����ʵķ����ᴿ����Һ�����ơ�������ԭ��Ӧ�ζ��ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȡ�
(1) �����������÷�Χ���÷��뾧������ϴ��׳����������ײ��ij������������ٹ��˵�ʱ��Ͳ������Ƚϼ�(2) �Բ�����е���Һ������������ٽ�����һ�������������ڲ������Һ�д��ڹ����Ĺ������⣬�����������һ���������ԣ���͢��м���IJ��ᷢ����Ӧ�����Լ�����е�Ŀ���dz�ȥ������˫��ˮ����߱���H2C2O4��Һ����������(3)���������Ҵ�ʹ������ֽᾧ�ɸ���Ŀ�������������������쵼�¾�����С��(4)��Ϊ��Ʒ�������Ҵ�����ѡ��95%���Ҵ����óɱ���ͣ���ѡC��(5) �ø�װ����ɾ���ij��˺�ϴ�ӵĹ��̣����ȿ������ã�Ȼ��ת�ƹ�������رջ���A��ȷ�ϳ�ɺ����A������ϴ�Ӽ�ϴ�ӣ�Ȼ���ٹػ���A��ȷ�ϳ�ɺ����A���ٹرճ����ã��ʴ�Ϊ��bdce�� (6)����������ܱ����Ը�������������ɶ�����̼�����ӷ���ʽΪ��16H++2MnO4-+5C2O42-=2Mn2++10CO2��+8H2O��
(7) 5K3[Fe(C204)3]��3H2O-- 6KMnO4
491*5 6
m 0.01��0.024��![]()
![]()
��m=0.982g�����Ʒ�Ĵ���=0.982/1.000=98.20%��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�