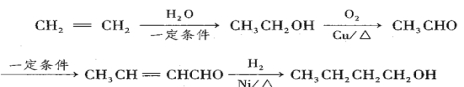��Ŀ����
����Ŀ���л���P��ij������ҩ����м��壬����һ�ֺϳ�·������
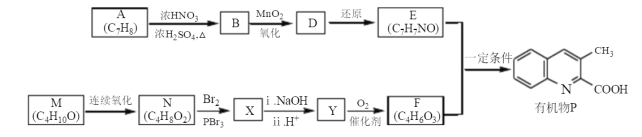
��֪��X�ķ���ʽΪC4H7O2Br
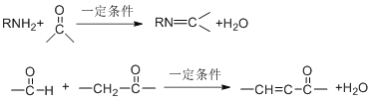
(1)�л���P�ķ���ʽΪ ________________��N��X�ķ�Ӧ������ ________________
(2)M��֧����Mϵͳ����������Ϊ____________��E�к��еĹ����ŵ�������_______________
(3)X��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ____________________________
(4)ij����K��E��ͬϵ�����Է���������E��14����ͬʱ������������K��ͬ���칹�干��_______�֡�
a.���б��� b.���ܷ���������Ӧ���ܷ���ˮ�ⷴӦ
д�������к���5�ֲ�ͬ��ѧ������Ľṹ��ʽ____________________
(5)����ϩΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�M��д���ϳ�·��(�ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)________________________________��
���𰸡�C11H9NO2 ȡ����Ӧ1-����ȩ�� ���� 5
5
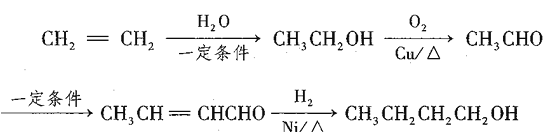
��������
��A�ķ���ʽ֪AΪ�ױ�����Ũ�����Ũ���������·���������Ӧ�����������ױ����ٱ�MnO2����ΪD����������ȩ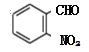 ������������ԭΪE
������������ԭΪE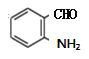 ����M�ķ���ʽC4H10O��M��֧����֪MΪ��������������������֪NΪ���ᣬ����֪����
����M�ķ���ʽC4H10O��M��֧����֪MΪ��������������������֪NΪ���ᣬ����֪����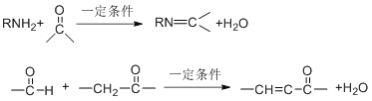 ��P��E�Ľṹ��֪FΪ
��P��E�Ľṹ��֪FΪ
CH3CH2COCOOH����F���ƿ�֪YΪCH3CH2CH(OH)COOH������Y���ƿ�֪X(����ʽΪC4H7O2Br)�Ľṹ��ʽΪCH3CH2CHBrCOOH��
(1) �����л���P�Ľṹ֪P�ķ���ʽΪC11H9NO2����X��Y��ת��������֪��X������±������ˮ�ⷴӦ���پ��ữ�õ�Y��Y�����м����Ȼ������ǻ���Y������������F��F�ķ����м����ʻ������Ȼ������N�ķ���ʽ��C4H8O2����F�ķ���ʽ��֪��N��X�ķ�Ӧ������ȡ����Ӧ���𰸣�C11H9NO2 ��ȡ����Ӧ��
(2) M�ķ���ʽΪC4H10O��M��֧����������������������Mϵͳ����������Ϊ1-��������P�Ľṹ����֪
![]() ��F�Ľṹ��ʽCH3CH2COCOOH�Ƴ�E�к��еĹ�������ȩ�� �������𰸣�ȩ�� ������
��F�Ľṹ��ʽCH3CH2COCOOH�Ƴ�E�к��еĹ�������ȩ�� �������𰸣�ȩ�� ������
(3) X�ķ���ʽΪC4H7O2Br��X������1-������������������巢����ȡ����Ӧ������
X��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ
(4)ij����K��E��ͬϵ�����Է���������E��14��Kͬʱ��������������a.���б�����b.���ܷ���������Ӧ���ܷ���ˮ�ⷴӦ�ģ�˵�����������ȩ��������������K��ͬ���칹����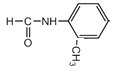 ���ƶ�ȡ�������ڡ��䡢��3�֣���
���ƶ�ȡ�������ڡ��䡢��3�֣���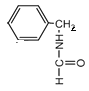
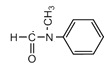 ������5�֡�
������5�֡�
�����к���5�ֲ�ͬ��ѧ������Ľṹ��ʽ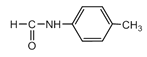 ���𰸣�5
���𰸣�5  ��
��
(5)��ΪM�ķ���ʽΪC4H10O��M��֧����������������������Mϵͳ����������Ϊ1-��������������ϩΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�M�ĺϳ�·��Ϊ��