��Ŀ����
(14��) ��A��B��C��D��E ����Ԫ�ص�ԭ��������������B��C ����������A�������Ӻ���ԭ�ӵĵ��Ӳ�ṹ��ͬ��A��B���γ����ӻ�����B2A��C�������������ǿ�ᷴӦ��������ǿ�Ӧ��D��ԭ�ӽṹʾ��ͼΪ��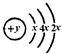 ��E�������������ǵ��Ӳ�����2�����Իش����и����⣺
��E�������������ǵ��Ӳ�����2�����Իش����и����⣺
��1��B��DԪ�طֱ�Ϊ �� ��
��2��DԪ��λ��Ԫ�����ڱ��е� ���ڡ��� �壻
��3��������B2A�ĵ���ʽ____________________��
��4��E������������ˮ����ķ���ʽ��________________________ ��
��
��5��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��6��C����������E ������������ˮ������Һ��Ӧ�����ӷ���ʽ��
������������ˮ������Һ��Ӧ�����ӷ���ʽ��
_______________________________________________________________________��
 ��E�������������ǵ��Ӳ�����2�����Իش����и����⣺
��E�������������ǵ��Ӳ�����2�����Իش����и����⣺��1��B��DԪ�طֱ�Ϊ �� ��
��2��DԪ��λ��Ԫ�����ڱ��е� ���ڡ��� �壻
��3��������B2A�ĵ���ʽ____________________��
��4��E������������ˮ����ķ���ʽ��________________________
 ��
����5��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��6��C����������E
 ������������ˮ������Һ��Ӧ�����ӷ���ʽ��
������������ˮ������Һ��Ӧ�����ӷ���ʽ��_______________________________________________________________________��
��1��B��Na ��D��Si ��2�֣�
��2���� IVA ��2�֣�
��3��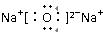 ��2�֣�
��2�֣�
��4��H2SO4 ��2�֣�
��5��Si + 2NaOH + H2O = Na2SiO3 + 2H2����3�֣�
��6��Al2O3 + 6H��= 2Al3��+ 3H2O��3�֣�
��2���� IVA ��2�֣�
��3��
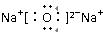 ��2�֣�
��2�֣���4��H2SO4 ��2�֣�
��5��Si + 2NaOH + H2O = Na2SiO3 + 2H2����3�֣�
��6��Al2O3 + 6H��= 2Al3��+ 3H2O��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ


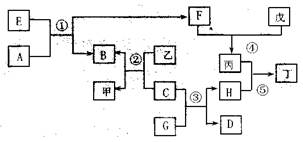
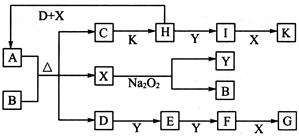
 2I��g����
2I��g����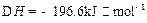 ��������4 mol H��2 mol Y�ų�345 kJ������ʱ��H��ת������ӽ���__________������ĸ����
��������4 mol H��2 mol Y�ų�345 kJ������ʱ��H��ת������ӽ���__________������ĸ����