��Ŀ����
����Ŀ�����ſ�ѧ�����ķ�չ�������ӵ������IJⶨ�ֶ�Խ��Խ�࣬�ⶨ����ҲԽ��Խ�ߣ�����һ�ּ��еIJⶨ���������岽��Ϊ��
�ٽ�����NaCl��ϸ�������ȷ��ȡmgNaCl����ת�Ƶ���������A�У�
���õζ�����A�����еμӱ����������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪVcm3��
(1)�������A���������__________��(����������)
(2)�����������ʽ�ζ��ܺû����ü�ʽ�ζ��ܺ�__________��������__________________��
(3)�ܷ��ý�ͷ�ιܴ��沽����еĵζ���__________��������_________________________
(4)�ܷ���ˮ���汽__________��������____________________
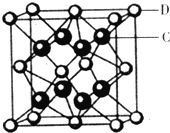
(5)��֪NaCl����ṹ��ͼ��ʾ����X���߲��NaCl�����п��������Na+��Cl-���ƽ������Ϊacm�����������ⶨ������õİ����ӵ�����NA�ı���ʽΪNA=__________��
���𰸡� ����ƿ ��ʽ�ζ��� ���ڱ����������͡��ϻ����ã����Եζ�������ʽ�ζ��� ���� ���ڽ�ͷ�ιܵ����� ���� ����ˮ���汽��NaCl���ܽ⣬���NaCl���������ȷ�ⶨ���� 58.5V/2ma3
����������1����������Ϊ����ƿ������һ������ұ��������Բ������A�������������ƿ����2�������и�ʴ�ԣ���ʴ��ʽ�ζ����е���Ƥ�ܣ�ֻ������ʽ�ζ��ܡ���3�����ڽ�ͷ�ιܵ��������Բ����ý�ͷ�ιܴ��沽����еĵζ�������4������mgNaCl���������������ܼ������ܽ�NaCl�����������NaCl����������Բ�����ˮ��Ӧ�ñ�����5��NaCl���ܶ�Ϊ![]() ��NaCl���������Ϊ��2a��3cm3����NaCl����������Ϊ
��NaCl���������Ϊ��2a��3cm3����NaCl����������Ϊ![]() ����2a��3cm3��
����2a��3cm3��![]() ��һ��NaCl������4����NaCl������ÿ����NaCl��������Ϊ
��һ��NaCl������4����NaCl������ÿ����NaCl��������Ϊ![]() ����
����![]() ��4��
��4��![]() �����NA=
�����NA=![]() ��
��

����Ŀ����ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�����������Ӱ�ˮ���������ܽ⣬�õ�����ɫ������Һ�������뼫�Խ�С���ܼ�(���Ҵ�)������������ɫ�ľ��塣
(��)�ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
(1)д����![]() ���ӻ�Ϊ�ȵ������������1�֣�__________________��
���ӻ�Ϊ�ȵ������������1�֣�__________________��
(2)ˮ�������ض����������õ�һ��![]() ���γ�ˮ�������ӣ�
���γ�ˮ�������ӣ�![]() �������ж��������̵���������������__________________��
�������ж��������̵���������������__________________��
A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |
(3)![]() �����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ_____________
�����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ_____________
(��)�������������Ʋ�����Һ����Ҫԭ�ϣ�������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϡ�
(4)д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ����ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ����(��Ԫ�ط���)��
(5)��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�����������Ӱ�ˮ���������ܽ⣬�õ�����ɫ������Һ�����Ϲ����з��������ӷ�Ӧ����ʽΪ��
_______________________________��_______________________________��ʵ��ʱ�γɵ�����ɫ��Һ�е��������ڴ��ڵ�ȫ����ѧ��������___________________��
(6)ʵ������м���![]() ��ɹ۲쵽��������ɫ
��ɹ۲쵽��������ɫ![]() ���塣ʵ��������
���塣ʵ��������![]() ��������__________________________________________________
��������__________________________________________________