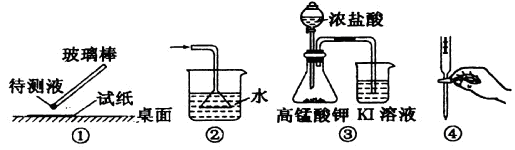
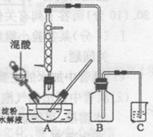
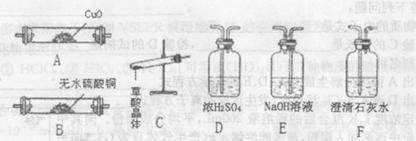
��Ŀ����
ij�о���ѧϰС������ữ����������Һ����I�����ڵĺ����Բ�������Ȥ��ͬѧ�Ǹ��ݱ�������˼��������·�����Ʋ�������ʵ��̽����
[�������]
����1�����ɵ�AgI������ˮ�����ܱ�HNO3������
����2��HNO3�������ԣ��ܽ�I��������I2��
[���ʵ�鷽������֤����]
��1����ͬѧ��KI��Һ�еμ����ữ��AgNO3��Һ�����л�ɫ�������ɡ���֤�˼���1��������д���йػ�ѧ����ʽ ��
��2����ͬѧ���ʵ����֤2�����������±������ݡ�
[���������]
��3����ͬѧ��֤�˼���1����������ͬѧ��֤�˼���2���������ͬѧ�ɴ˵ó��Ľ��ۣ������ữ��AgNO3����ʹI��������Ҳ������I������ͬ���ͬѧ�Ľ����𣬲��������ɣ� ��
[˼���뽻��]
��3���о���ѧϰС��ͬѧ�����о�Fe3+�ܷ�����I�������⣬���������ַ���������ʵ����֤��
����һ��
��������
������֤��������д����Ҫ���ӷ���ʽ ��
[�������]
����1�����ɵ�AgI������ˮ�����ܱ�HNO3������
����2��HNO3�������ԣ��ܽ�I��������I2��
[���ʵ�鷽������֤����]
��1����ͬѧ��KI��Һ�еμ����ữ��AgNO3��Һ�����л�ɫ�������ɡ���֤�˼���1��������д���йػ�ѧ����ʽ ��
��2����ͬѧ���ʵ����֤2�����������±������ݡ�
| ʵ�鲽�裨��Ҫ��д����������̣� | Ԥ������ͽ��� |
| | ����Һ����������2������ ����Һ������������2�������� |
| ���� |
[���������]
��3����ͬѧ��֤�˼���1����������ͬѧ��֤�˼���2���������ͬѧ�ɴ˵ó��Ľ��ۣ������ữ��AgNO3����ʹI��������Ҳ������I������ͬ���ͬѧ�Ľ����𣬲��������ɣ� ��
[˼���뽻��]
��3���о���ѧϰС��ͬѧ�����о�Fe3+�ܷ�����I�������⣬���������ַ���������ʵ����֤��
����һ��
��������
������֤��������д����Ҫ���ӷ���ʽ ��
(12�֣�ÿ��2��)
��1��KI+AgNO3=AgI��+KNO3
��2��ȡ��ͬѧ���õ������ữ��AgNO3�μӵ�KI��������Һ��
��3����ͬ�⣬�����ữ��AgNO3���������ᣬ����֤�������Ƿ�������I-
��4������һ����FeCl3��Һ�еμӼ���KI��������Һ������Һ�Ƿ����
����������KI��KSCN��Һ�еμӼ���FeCl3��Һ������Һ�Ƿ��ȱ�����ɫ2Fe3++2I-=I2+2Fe2+
��1��KI+AgNO3=AgI��+KNO3
��2��ȡ��ͬѧ���õ������ữ��AgNO3�μӵ�KI��������Һ��
��3����ͬ�⣬�����ữ��AgNO3���������ᣬ����֤�������Ƿ�������I-
��4������һ����FeCl3��Һ�еμӼ���KI��������Һ������Һ�Ƿ����
����������KI��KSCN��Һ�еμӼ���FeCl3��Һ������Һ�Ƿ��ȱ�����ɫ2Fe3++2I-=I2+2Fe2+
�����������2�����������ǿ�����Ժ͵�ʹ���۱���ɫ�����ԣ���4���������֤ʵ�鷽��ʱҪע�����һ�����������ǵ�����Ϊ��֤�������Ƿ���I2���Ƿ���Fe3+��

��ϰ��ϵ�д�
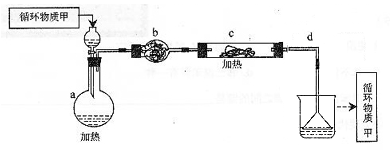
�����Ŀ


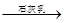 Mg��OH��2
Mg��OH��2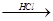 MgCl2��Һ��MgCl2��6H2O��MgCl2
MgCl2��Һ��MgCl2��6H2O��MgCl2 Mg
Mg