��Ŀ����
����Ŀ��2019��9�£��ҹ�������Ա���Ƴ�Ti��H��Fe˫��������������Ti��H�����Fe������¶Ȳ�ɳ���100����Ti��H��Fe˫�������ϳɰ��ķ�Ӧ������ͼ��ʾ�����������ڴ��������ϵ�������*��ע������˵��������� (����)
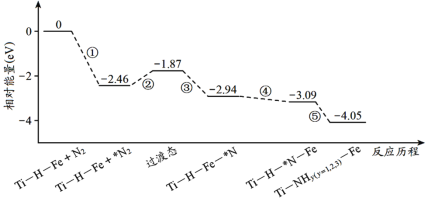
A.�٢ڢ��ڸ������������ܢ��ڵ���������
B.�������������仯������2.46eV���ǵ������е��������Ķ��ѹ���
C.�ڸ������ӿ��˷�Ӧ���ʣ�����������˰��IJ���
D.ʹ��Ti��H��Fe˫�������ϳɰ�������ı�ϳɰ���Ӧ�ķ�Ӧ��
���𰸡�B
��������
A����Ϊ��������N2�Ĺ��̣���Ϊ�γɹ���̬�Ĺ��̣���ΪN2����ΪN�Ĺ��̣����϶���Ҫ�ڸ���ʱ���У�Ŀ���Ǽӿ췴Ӧ���ʣ����ܢ�Ϊ������ƽ����ʣ���Ҫ�ڵ����½��У���A��ȷ��
B����ͼ��֪�������������仯������2.46eV���ù���ΪN2���������̣���������û�ж��ѣ���B����
C�������¶ȿ���߷�Ӧ���ʣ����Ը������ӿ��˷�Ӧ���ʣ����ϳɰ��ķ�ӦΪ���ȷ�Ӧ�����Ե���������߰��IJ��ʣ���C��ȷ��
D�������ܸı䷴Ӧ���̣����ͷ�Ӧ�Ļ�ܣ������ܸı䷴Ӧ��ʼ̬����̬�������ܸı䷴Ӧ�ķ�Ӧ�ȣ���D��ȷ��
�ʴ�ΪB��





