��Ŀ����
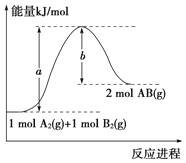
����Ŀ����ͼ��298 KʱN2��H2��Ӧ�����������仯������ͼ������������ȷ����
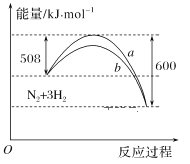
A.���¶ȡ����һ���������£�ͨ��1molN2��3molH2��Ӧ��ų�������ΪQ1kJ����ͨ��2molN2��6mol H2��Ӧ��ų�������ΪQ2kJ����184>Q2>2Q1
B.�÷�Ӧ���Ȼ�ѧ����ʽΪ��N2��3H22NH3 ��H����92kJ��mol��1
C.a�����Ǽ������ʱ�������仯����
D.����������û�ѧ��Ӧ�ķ�Ӧ�ȸı�
���𰸡�A
��������
A. ���ܱ�������ͨ��1molN2��3molH2����������ȫת����Q1С��92 kJ�����Դﵽƽ��ʱ�ų���������һ�ݻ���ͬ���ܱ�������ͨ��2molN2��6mol H2���ﵽƽ��ʱ�ų����������Ͻ�Ӧ����2��92 kJ=184 kJ������Ӧ�ﲻ���ܳ�ֽ��г��ף�����Q2С��184 kJ��ͬʱͨ��2molN2��6mol H2��ѹǿ��ͨ��1mol N2��3mol H2�Ĵ�ƽ����������ƶ����ų���������������Q2>2Q1����184>Q2>2Q1����A��ȷ��
B. �Ȼ�ѧ����ʽ�����ע���ʵľۼ�״̬��Ӧ���ʱ䣬�÷�Ӧ���Ȼ�ѧ����ʽΪ��N2��3H22NH3 ��H����92kJ��mol��1����B����
C.�����ܸı䷴Ӧ��·����ʹ������Ӧ����Ļ�ܽ��ͣ������ı仯ѧƽ�⣬��Ӧ����ЧӦ���䣬��ͼ���е�b�����Ǽ���������ʱ�������仯���ߣ���C����
D. �����ܸı䷴Ӧ��·����ʹ������Ӧ����Ļ�ܽ��ͣ������ı仯ѧƽ�⣬��Ӧ����ЧӦ���䣬��D����
��ѡA��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬ�����������գ�
(1)����100mL 0.10mol��L-1NaOH����Һ��
����Ҫ�������裺�������������ܽ���(��ȴ��)___��ϴ��(����ϴ��Һ��������ƿ)��___��____�������ƺõ���Һ�����Լ�ƿ�У����ϱ�ǩ��
�ڳ���__________g�������ƹ��壬���������У���ƽ(�����롢����)��_______��________��
(2)ȡ20.00mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�ҺNaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡�
ʵ���� | NaOH��Һ��Ũ��(mol/L) | �ζ���ɺ�NaOH��Һ��������(mL) | ������������(mL) |
1 | 0.10 | 22.64 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.80 | 20.00 |
�ٵζ��ﵽ�յ�ı�־��___________________________��
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ__________(������λ��Ч����)��
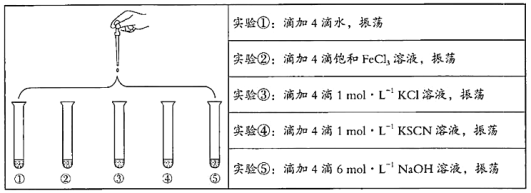
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е�__________��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ���__________��
A.�ζ��յ����ʱ���Ӷ���
B.��ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C.��ƿˮϴ��δ����
D.��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������з�Ӧ��2N2O5(g) ![]() 4NO2(g) + O2(g) ��H ��+Q kJ/mol (Q>0)��ij�¶��£���2L���ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
4NO2(g) + O2(g) ��H ��+Q kJ/mol (Q>0)��ij�¶��£���2L���ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
ʱ��/s | 0 | 500 | 1000 | 1500 |
c(N2O5)/mol/L | 5.0 | 3.5 | 2.5 | 2.5 |
����˵����ȷ����
A. 500s��N2O5�ֽ�����Ϊ6��10-3mol/(L��s)
B. ���¶��µ�ƽ�ⳣ��K ��125
C. ��Ӧ��ƽ��ʱ�����յ�����Ϊ5Q kJ
D. �����������䣬����ʼʱc(N2O5)��10mol/L�����ƽ���c(N2O5)��5mol/L
����Ŀ������˵������ȷ���ǣ� ��
A.HF��HCl��![]() ��
��![]() ���ȶ���������ǿ
���ȶ���������ǿ
B.��Mg��Si��N��F��˳��ԭ�Ӱ뾶��С���
C.ij����Ԫ�صĵ�����![]() �������±���ʾ
�������±���ʾ![]() ��λ��
��λ��![]() �����Ʋ��Ԫ��λ��Ԫ�����ڱ��ڢ�A��
�����Ʋ��Ԫ��λ��Ԫ�����ڱ��ڢ�A��
I | I | I | I | I | I | I |
578 |
|
|
|
|
|
|
D.�ڢ�P��S����![]() ��Ca����
��Ca����![]() ��Si����Ԫ���У�ÿ���е�һ�����ܽϴ��Ԫ�ص�ԭ������֮��Ϊ41
��Si����Ԫ���У�ÿ���е�һ�����ܽϴ��Ԫ�ص�ԭ������֮��Ϊ41