��Ŀ����
|
��һ�̶��ݻ����ܱ������з������·�Ӧ�� aX(g)��bY(g) | |
| [����] | |
A�� |
3��1��2��1 |
B�� |
1��3��2��2 |
C�� |
1��3��1��2 |
D�� |
3��1��2��2 |
������
|
��������֮�ȵ������ʵļ�����֮�ȣ���ȷ�� X��Y��Z�ļ�����֮��Ϊ3��1��2������Ϊ��Ӧǰ��ѹǿ���ֲ��䣬˵����Ӧǰ��������ܵ����ʵ������䣬���Ʋ�W�ļ�����Ϊ2�� |

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д����Ǵ����к����ḻ��һ��Ԫ�أ������仯������������������������Ҫ���ã����ٵ���������ŷ��ǻ�����������Ҫ����֮һ����ش����е����仯�����������⣺
(1)�ݱ������������ѧ�һ���˼����о���ֵ��N4������ӽṹ������ӵ���������ṹ���ơ���֪����1 mol N��N������167 kJ����������1 mol N��N���ų�942 kJ��������д��N4����ת��ΪN2��Ӧ���Ȼ�ѧ����ʽ�� ��
(2)�ݱ�����NH3��ֱ����������ȼ�ϵ�أ�д���õ�صĸ�����Ӧʽ�� ��
(3)��T1��ʱ����5 mol N2O5����10L�̶��ݻ����ܱ������з������з�Ӧ��2N2O5(g) 4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%��
4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%��
����÷�Ӧ��ƽ�ⳣ��K= (���ִ���ʽ�Ӽ���)������ƽ����ϵ��O2���������Ϊ__________��
����O2��ʾ��0��5 min�ڸ÷�Ӧ��ƽ�����ʦ�(O2)= ��
�۽�����ƽ����ϵ���¶Ƚ���T2�棬�ܱ������ڼ�С���������� ��
A��ѹǿ B���ܶ� C����Ӧ���� D��N2O5��Ũ��
(4)�ں��º��ݵ��ܱ������г���NO2����������ƽ�⣺2NO2(g) N2O4(g)��ƽ��ʱN2O4��NO2�����ʵ���֮��Ϊa�������������������£��ֱ��ٳ���NO2���ٳ���N2O4��ƽ�������ı仯��ȷ����__________��
N2O4(g)��ƽ��ʱN2O4��NO2�����ʵ���֮��Ϊa�������������������£��ֱ��ٳ���NO2���ٳ���N2O4��ƽ�������ı仯��ȷ����__________��
A��������a��С B��������a���� C������NO2����a��С������N2O4����a����
D������NO2����a������N2O4����a��С
 ���ǵ����Ϻ����ḻ��Ԫ�أ������仯������о���������������������Ҫ���壮
���ǵ����Ϻ����ḻ��Ԫ�أ������仯������о���������������������Ҫ���壮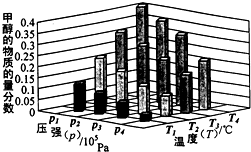 ��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ�
��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ� 4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%��
4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%��