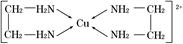
��Ŀ����
��1������Ԫ��A�ļ������Ӳ����κ�ԭ�Ӿ�����ͬ�ĺ�������Ų���Ԫ��B�뵪Ԫ��ͬ���ڣ�B��ԭ���������ڵ�������һ�����ܱȵ���С��A��B���γ����ֻ�����A2B2��A2B������B���ӻ���ʽ�ֱ�Ϊ �� ��A2B��NH3��SiH4�ļ����ɴ�С����Ϊ ���ѧʽ����A2B��Һ̬�γɾ���ʱ�ܶȼ�С����Ҫԭ���� ��
��2���������������������㷺Ӧ�á��մɷ������IJ���ѡ�õ����裬��Ӳ�ȴ�ѧ�ȶ���ǿ���Ǻܺõĸ����մɲ��ϡ���������⣬�����費���������ᷴӦ������ʴ����ǿ��������ľ��������� ��������������ᷴӦ�Ļ�ѧ����ʽΪ ��
��3��MgC03��CaC03��Ϊ���Ӿ��壬�ȷֽ���¶ȷֱ�Ϊ402���900�棬����ݽṹ�����ʵĹ�ϵ˵�������ȷֽ��¶Ȳ�ͬ��ԭ�� ��
��4���黯�ع㷺�����״���Ӽ�������������ǡ�����ɴ��ȼ�˼����С��صĻ�̬ԭ�Ӽ۵����Ų�ʽΪ ���黯�صľ����ṹ����ʯ���ƣ��侧���߳�Ϊa pm����ÿ�������þ�����������Ԫ�ص�����Ϊ g����NA��ʾ�����ӵ���������
��2���������������������㷺Ӧ�á��մɷ������IJ���ѡ�õ����裬��Ӳ�ȴ�ѧ�ȶ���ǿ���Ǻܺõĸ����մɲ��ϡ���������⣬�����費���������ᷴӦ������ʴ����ǿ��������ľ��������� ��������������ᷴӦ�Ļ�ѧ����ʽΪ ��
��3��MgC03��CaC03��Ϊ���Ӿ��壬�ȷֽ���¶ȷֱ�Ϊ402���900�棬����ݽṹ�����ʵĹ�ϵ˵�������ȷֽ��¶Ȳ�ͬ��ԭ�� ��
��4���黯�ع㷺�����״���Ӽ�������������ǡ�����ɴ��ȼ�˼����С��صĻ�̬ԭ�Ӽ۵����Ų�ʽΪ ���黯�صľ����ṹ����ʯ���ƣ��侧���߳�Ϊa pm����ÿ�������þ�����������Ԫ�ص�����Ϊ g����NA��ʾ�����ӵ���������
��1��sp3(1��) sp,3(1��)SiH4>NH3>H2O��2�֣��γɾ���ʱ��ÿ��ˮ������4��ˮ�����γ���������ɿռ�����������״�ṹ��ˮ���ӵĿռ������ʵͣ��ܶȷ�����С��2�֣�
��2��ԭ�Ӿ���(1��)Si3N4+12HF=3SiF4+4NH3����2�֣�
��3��Mg2+�뾶С��Ca2+�뾶����MgO�����ܴ���CaO�����ܣ�����Mg2+��Ca2+������̼��������е������ӽ�ϣ�ʹ̼������ӷֽ�Ϊ������̼����2�֣�
��4��4s24p1��2�֣� 3.00��1032/a3��NA��2�֣�
��2��ԭ�Ӿ���(1��)Si3N4+12HF=3SiF4+4NH3����2�֣�
��3��Mg2+�뾶С��Ca2+�뾶����MgO�����ܴ���CaO�����ܣ�����Mg2+��Ca2+������̼��������е������ӽ�ϣ�ʹ̼������ӷֽ�Ϊ������̼����2�֣�
��4��4s24p1��2�֣� 3.00��1032/a3��NA��2�֣�
���������(1) ����Ԫ��A�ļ������Ӳ����κ�ԭ�Ӿ�����ͬ�ĺ�������Ų�,��A��HԪ�أ�A��B���γ����ֻ�����A2B2��A2B��BΪ�ڶ�����Ԫ�أ�����BΪOԪ�أ�������A2B2��A2B�ֱ���H2O2��H2O�����߶���sp3�ӻ���SiH4��NH3��H2O�ļ۲���ӶԶ���4�����µ��Ӷ����ֱ���0��1��2���۲���Ӷ�����ͬʱ�¶�Խ�����ԽС�����Լ��ǵĴ�С˳����SiH4>NH3>H2O��Һ̬ˮ���ܶȴ��ڱ�������Ϊ���е�ÿ��ˮ������4��ˮ�����γ���������ɿռ�����������״�ṹ��ˮ���ӵĿռ������ʵͣ��ܶȷ�����С��
��2��������Ӳ�ȴ�ѧ�ȶ���ǿ������ԭ�Ӿ��壻������������ᷴӦ�����ķ�����Ͱ�������ѧ����ʽΪSi3N4+12HF=3SiF4+4NH3����
��3�����ߵ������Ӷ���̼������ӣ������Ӳ�ͬ������Ӧ�Ƚ������ӵİ뾶�IJ�ͬ�����������Ӽ���ǿ����ͬ��Mg2+�뾶С��Ca2+�뾶����MgO�����ܴ���CaO�����ܣ�����Mg2+��Ca2+������̼��������е������ӽ�ϣ�ʹ̼������ӷֽ�Ϊ������̼��
��4�����ǵ������ڢ�A����Ԫ�أ������3�����ӣ��������̬ԭ�Ӽ۵����Ų�ʽΪ4s24p1���黯�صľ����ṹ����ʯ���ƣ������黯�صľ�������4����ԭ�ӣ���1����������Ԫ�ص�������
4��75g/NA=300g/NA�����������Ϊ��a��10-10��3cm3,����ÿ�������þ�����������Ԫ�ص�����Ϊ3.00��1032/a3��NAg��

��ϰ��ϵ�д�
�����Ŀ
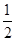 ������˵����ȷ����
������˵����ȷ����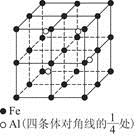


 ����
����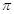 ����Ŀ֮��Ϊ ��
����Ŀ֮��Ϊ ��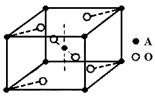
 2CuCl����4H����SO42-
2CuCl����4H����SO42-