��Ŀ����
X��Y��Z��M��WΪ���ֶ���������Ԫ�ء�X��Y��Z��ԭ���������ε�����ͬ����Ԫ�أ�������������֮��Ϊ15��X��Z���γ�XZ2���ӣ�Y��M�γɵ���̬�������ڱ�״���µ��ܶ�Ϊ0��76g��L��1��W����������X��Y��Z��M����Ԫ��������֮�͵�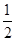 ������˵����ȷ����
������˵����ȷ����
 ������˵����ȷ����
������˵����ȷ����| A��ԭ�Ӱ뾶��W��Z��Y��X��M |
| B��XZ2��X2M2��W2Z2��Ϊֱ���ι��ۻ����� |
| C����XԪ���γɵĵ��ʲ�һ����ԭ�Ӿ��� |
| D����Y��Z��M����Ԫ���γɵĻ�������ֻ���й��ۼ� |
C
����������������������֪��X��Y��Z��M��W�����ֶ�����Ԫ�ص����У����ǰ�ԭ���������ε������еģ�����ֻ��X��Y��Z����Ԫ����ԭ���������ε�����ͬ����Ԫ�أ���X��Y��Z������������֮��Ϊ15��X��Z���γ�XZ2���ӣ����Ƴ�X��Y��Z�ֱ�ΪC��N��O����Ԫ�أ�����Y��M�γɵ���̬�������ڱ�״���µ��ܶ�0��76g?L-1���Ϳɼ��������̬�������Ħ������Ϊ22��4L/mol��0��76g?L-1=17g/mol���Ӷ�ȷ��MΪHԪ�أ�����W����������X��Y��Z��M����Ԫ��������֮�͵�
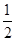 ���Ƴ�W��������Ϊ
���Ƴ�W��������Ϊ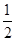 ����6+7+8+1��=11������WΪNaԪ�أ���A��ԭ�Ӱ뾶Ӧ��W��X��Y��Z��M����Na��C��N��O��H������A����B��ѡ����CO2��C2H2��Ϊֱ�����ۻ������Na2O2�����ӻ��������ֱ�����ۻ������B����C��̼��ͬ��������ܶ࣬���ʯΪԭ�Ӿ��壬������ʯī��C60��̼���ܡ�ʯīϩ��̼���ʾͲ���ԭ�Ӿ��壬��C��ȷ��D��Y��Z��M����Ԫ�ؿ��γɻ���������泥��������ӻ�����������Ӽ�����D����ѡC��
����6+7+8+1��=11������WΪNaԪ�أ���A��ԭ�Ӱ뾶Ӧ��W��X��Y��Z��M����Na��C��N��O��H������A����B��ѡ����CO2��C2H2��Ϊֱ�����ۻ������Na2O2�����ӻ��������ֱ�����ۻ������B����C��̼��ͬ��������ܶ࣬���ʯΪԭ�Ӿ��壬������ʯī��C60��̼���ܡ�ʯīϩ��̼���ʾͲ���ԭ�Ӿ��壬��C��ȷ��D��Y��Z��M����Ԫ�ؿ��γɻ���������泥��������ӻ�����������Ӽ�����D����ѡC��
��ϰ��ϵ�д�
�����Ŀ
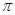 ����___________mol��
����___________mol��