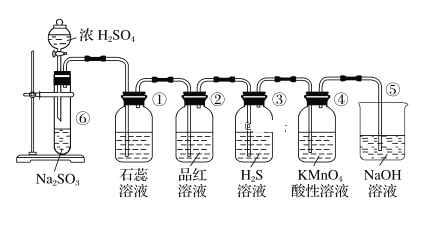
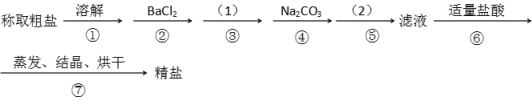
��Ŀ����
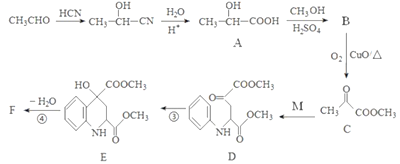
����Ŀ��ҩ��F���п���������Ѫ�ǡ���Ѫѹ�ȶ���������ԣ���ϳ�·�����£�
��֪��M�Ľṹ��ʽΪ�� ��
��
��ش��������⣺
(1)A�Ļ�ѧ������______________________��
(2)C�й����ŵ�������_________________________��
(3)д��F�Ľṹ��ʽ____________________________��
(4)��֪A��һ�������������ɿɽ���ľ�������д���÷�Ӧ��ѧ����ʽ��_________________��
(5)��������������M��ͬ���칹����_____��(���������칹)��
���ܹ�����������Ӧ��
�ں�������(-NO2)��������ֱ�����ڱ����ϡ�
�ۺ��б����ұ�����ֻ������ȡ������
���к˴Ź�������Ϊ������ҷ����֮��Ϊ6��2��2��1�Ľṹ��ʽΪ______________(д��һ�ּ���)��
(6)д������ȩΪԭ���Ʊ��߷��ӻ�����۱�ϩ��ĺϳ�·��(���Լ���ѡ)��_____________��
���𰸡� 2-�Ȼ�����(���-�Ȼ����������) ͪ�ʻ� ���� 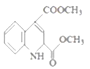
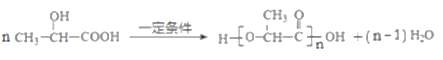 15
15 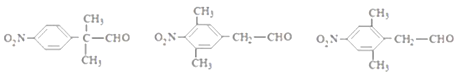
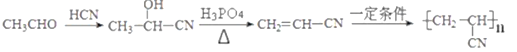
����������������ͼ����ȩ��HCN�����ӳ�����CH3CH(OH)-CN��CH3CH(OH)-CNˮ������CH3CH(OH)-COOH��A����CH3CH(OH)-COOH��״���������CH3CH(OH)-COOCH3 (B)��B��������CH3COCOOCH3����M��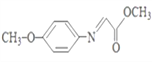 ����Ӧ����D ��
����Ӧ����D ��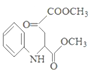 ����D����ԭ����E��E������ȥ��Ӧ����F���ṹ��ʽΪ
����D����ԭ����E��E������ȥ��Ӧ����F���ṹ��ʽΪ ��
��
(1)����A�Ľṹ��ʽCH3CH(OH)-COOH�����л������������࣬����Ϊ2-�Ȼ�����(���-�Ȼ����������)����ȷ�𰸣�2-�Ȼ�����(���-�Ȼ����������)��
(2)�л���CΪCH3COCOOCH3�������ŵ�������ͪ�ʻ��� ��������ȷ�𰸣�ͪ�ʻ��� ������
(3)�������Ϸ�����֪���л���F�Ľṹ��ʽΪ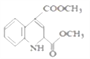 ����ȷ�𰸣�
����ȷ�𰸣� ��
��
(4)�л���AΪCH3CH(OH)-COOH����һ�������·������۷�Ӧ����Ӧ��ѧ����ʽ��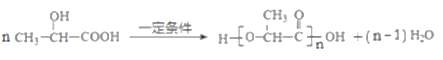 ����ȷ�𰸣�
����ȷ�𰸣�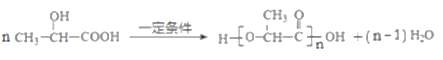 ��
��
(5)M��ͬ���칹�壺���ܹ�����������Ӧ������ȩ�����ں�������(-NO2)��������ֱ�����ڱ����ϣ��ۺ��б����ұ�����ֻ������ȡ���������������µ��л����DZ����Ϻ�������������һ������Ϊ-C3H6-CHO������-C3H6-CHO��-CH2CH2CH2CHO��-CH2CH(CH3)CHO���CCH(CH3)CH2CHO��-CH(CH2CH3)-CHO��-C(CH3)2CHO 5�ֽṹ�����M��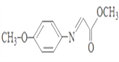 ����ͬ���칹����3��5=15�֣����к˴Ź�������Ϊ������ҷ����֮��Ϊ6��2��2��1�Ľṹ��ʽΪ
����ͬ���칹����3��5=15�֣����к˴Ź�������Ϊ������ҷ����֮��Ϊ6��2��2��1�Ľṹ��ʽΪ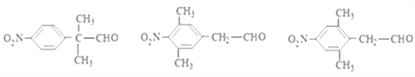 ����ȷ�𰸣�15��
����ȷ�𰸣�15��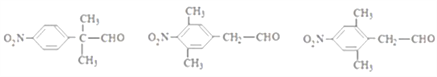 ��
��
(6)����ȩ�Ʊ��۱�ϩ�棨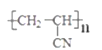 �������Ʊ���ϩ�棬��ȩ��HCN�ӳ�����CH3CH(OH)-CN��CH3CH(OH)-CN������ȥ���ɱ�ϩ������ϩ�淢���Ӿ����ɾ۱�ϩ�棻�ϳ�·��Ϊ��
�������Ʊ���ϩ�棬��ȩ��HCN�ӳ�����CH3CH(OH)-CN��CH3CH(OH)-CN������ȥ���ɱ�ϩ������ϩ�淢���Ӿ����ɾ۱�ϩ�棻�ϳ�·��Ϊ��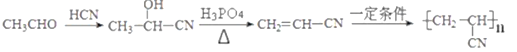 ����ȷ�𰸣�
����ȷ�𰸣�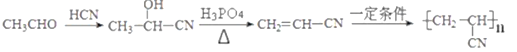 ��
��