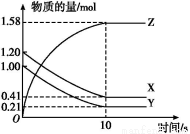
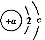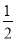МвДҝДЪИЭ
ФЪ298 KЎў1.01ЎБ105 PaПВ,Ҫ«22 g CO2НЁИл750 mL 1 molЎӨL-1NaOHИЬТәЦРід·Ц·ҙУҰ,ІвөГ·ҙУҰ·Еіцx kJөДИИБҝЎЈТСЦӘФЪёГМхјюПВ,1 mol CO2НЁИл1 L 2 molЎӨL-1 NaOHИЬТәЦРід·Ц·ҙУҰ·Еіцy kJөДИИБҝЎЈФтCO2УлNaOHИЬТә·ҙУҰЙъіЙNaHCO3өДИИ»ҜС§·ҪіМКҪОӘ(ЎЎЎЎ)
A.CO2(g)+NaOH(aq) NaHCO3(aq) ҰӨH=-(2y-x)kJЎӨmol-1
NaHCO3(aq) ҰӨH=-(2y-x)kJЎӨmol-1
B.CO2(g)+NaOH(aq) NaHCO3(aq) ҰӨH=-(2x-y)kJЎӨmol-1
NaHCO3(aq) ҰӨH=-(2x-y)kJЎӨmol-1
C.CO2(g)+NaOH(aq) NaHCO3(aq) ҰӨH=-(4x-y)kJЎӨmol-1
NaHCO3(aq) ҰӨH=-(4x-y)kJЎӨmol-1
D.CO2(g)+NaOH(aq) NaHCO3(aq) ҰӨH=-(8x-2y)kJЎӨmol-1
NaHCO3(aq) ҰӨH=-(8x-2y)kJЎӨmol-1
C
ЎҫҪвОцЎҝёщҫЭ·ҙУҰCO2+NaOH NaHCO3ЎўCO2+2NaOH
NaHCO3ЎўCO2+2NaOH Na2CO3+H2O,Фт22 g CO2НЁИл750 mL 1 molЎӨL-1 NaOHИЬТәЦРід·Ц·ҙУҰІъОпОӘNaHCO3ЎўNa2CO3өД»мәПОпЎЈЙиІОјУ·ҙУҰЙъіЙNaHCO3өДCO2ОпЦКөДБҝОӘa,ІОјУ·ҙУҰЙъіЙNa2CO3өДCO2ОпЦКөДБҝОӘb,Фтa+b=0.5 mol,a+2b=0.75 mol,ҪвөГ:a=b=0.25 molЎЈёщҫЭИИБҝБР№ШПө,ЙПКц·ҙУҰЦРІъЙъ0.25 mol NaHCO3·ЕіцөДИИБҝОӘ(x-0.25y)kJ,№КСЎCПоЎЈ
Na2CO3+H2O,Фт22 g CO2НЁИл750 mL 1 molЎӨL-1 NaOHИЬТәЦРід·Ц·ҙУҰІъОпОӘNaHCO3ЎўNa2CO3өД»мәПОпЎЈЙиІОјУ·ҙУҰЙъіЙNaHCO3өДCO2ОпЦКөДБҝОӘa,ІОјУ·ҙУҰЙъіЙNa2CO3өДCO2ОпЦКөДБҝОӘb,Фтa+b=0.5 mol,a+2b=0.75 mol,ҪвөГ:a=b=0.25 molЎЈёщҫЭИИБҝБР№ШПө,ЙПКц·ҙУҰЦРІъЙъ0.25 mol NaHCO3·ЕіцөДИИБҝОӘ(x-0.25y)kJ,№КСЎCПоЎЈ

 јЖЛгёЯКЦПөБРҙр°ё
јЖЛгёЯКЦПөБРҙр°ё