��Ŀ����
����Ŀ���� NaOH ��Na2CO3 �����Һ�еμ� 0.1 mol��L1 ϡ���ᣬCO2 ����������������������Ĺ�ϵ��ͼ�������ж���ȷ����( )
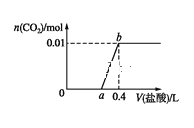
A.�� 0~a ��Χ�ڣ������кͷ�Ӧ�� CO32-+H+=HCO3-
B.ab �η�����Ӧ�����ӷ���ʽΪ�� CO32-+2H+ CO2 +H2O
C.a=0.2
D.ԭ�����Һ��NaOH �� Na2CO3 �����ʵ���֮��Ϊ 1��2
���𰸡�A
��������
��NaOH��Na2CO3�����ɵ���Һ�����μ�����ʱ�������������������ȷ�����Ӧ��NaOH��HCl��NaCl��H2O��Ȼ��̼���������ᷴӦ����̼�����ƺ��Ȼ��ƣ�HCl��Na2CO3��NaHCO3��NaCl�����̼�����������ᷴӦ���ж�����̼�������ɣ��������ʵ����Ĺ�ϵ�����ͼ�ɽ��
A���ɷ�����֪���� 0~a ��Χ�ڣ�û�в������壬�����кͷ�ӦNaOH��HCl��NaCl��H2O�������μ�����ʱ�������Na2CO3������ӦHCl��Na2CO3��NaHCO3��NaCl�����ӷ���ʽΪCO32-+H+=HCO3-����A��ȷ��
B��ab�η�����ӦΪ��NaHCO3��HCl��NaCl��H2O��CO2������Ӧ�����ӷ���ʽΪ��HCO3��H����H2O��CO2������B����
C������̼Ԫ���غ㣬�������̼�������ʵ�����0.01mol������������Ӧ��HCl��Na2CO3��NaHCO3��NaCl��NaHCO3��HCl��NaCl��H2O��CO2�������ĵ�HCl���ʵ�������0.01mol��0.1molL1ϡ���������ֱ���0.1L������a��0.3����C����
D������C��ļ��㣬��NaOH��Ӧ��HCl���ʵ���Ϊ0.02mol����ԭ�����Һ��NaOH��Na2CO3�����ʵ����ֱ���0.02mol��0.01mol�����ʵ���֮��Ϊ2��1����D����
�ʴ�ѡA��

����Ŀ����a��b��c��d�ĸ������缫���йصķ�Ӧװ�ü����ַ�Ӧ�������£�
ʵ��װ�� | ����ʵ������ |
| a����������b���������� |
| b�������������c���ޱ仯 |
| d���ܽ⣬c����������� |
| ������ָʾ�ڵ����е�����a������d�� |
�ɴ˿��ж������ֽ����Ļ��˳���ǣ���������
A.a>b>c>dB.b>c>d>aC.d>a>b>cD.a>b>d>c
����Ŀ������ʵ�����������ͽ��۾���ȷ����
ѡ�� | ʵ����� | ���� | ���� |
A | ��ͭ�ۼ���1.0mol/LFe2(SO4)3��Һ�� | ��Һ��Ϊ��ɫ | ��������ͭ���� |
B | ������ǯ��סһС����ɰֽ��ϸ��ĥ���������ھƾ����ϼ��� | �ۻ����Һ̬������������ | ���������۵���������۵� |
C | �����£���pH�Ʋ�0.1mol/LNaX��Һ��0.1mol/LNa2CO3��Һ��pH | ǰ��С�ں��� | ���ԣ�HX>H2CO3 |
D | ��10%��������Һ�м�������ϡ���ᣬˮԡ����һ��ʱ�䣬�ټ���������Һ | δ���ֹ������� | ����δ����ˮ�� |
A. A B. B C. C D. D