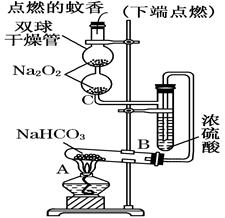
��Ŀ����
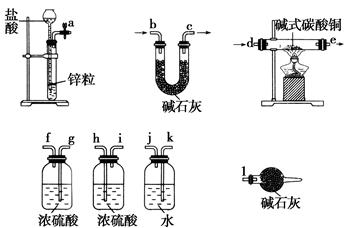
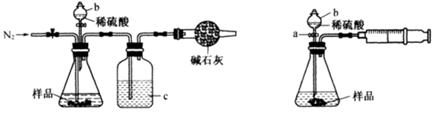
ij�о���ѧϰС�飬Ϊ��̽���������Ƶ�ǿ�����ԣ��������ͼ��ʵ��װ�á�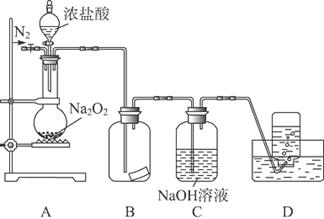
ʵ�鲽�輰�������£�
�ټ��װ�������Ժ�װ��ҩƷ������������
�ڻ���ͨ��һ������N2��װ��D���Ӻ�(����ĩ��δ���뼯��ƿ��)������Բ����ƿ�л����μ�Ũ���ᣬ��Ӧ���ң���������ɫ���塣
��һ��ʱ�������ĩ�����뼯��ƿ���ռ����塣װ��D���ռ�����ʹ�����ǵ�ľ����ȼ����ɫ���塣
�ܷ�Ӧ�����رշ�Һ©���Ļ�������ͨ��һ������N2����װ����������ɫ��
�ش��������⣺
��1��װ��B�е�ʪ��ĺ�ɫֽ����ɫ��֤��A�з�Ӧ��________(�ѧʽ)���ɡ���B�иķ�ʪ��ĵ���KI��ֽ����ƾ��ֽ������������֤���������ۣ��������ӷ���ʽ˵��ԭ��________________��
��2��װ��C��������_________________________________________________________��
��3����ͬѧ��ΪO2��Na2O2�������е�HCl��ԭ���á���ͬѧ��Ϊ�˽��۲���ȷ�������ܵ�����Ϊ��_________________________________________________________________��
��______ __��
��4��ʵ��֤����Na2O2��������HCl��Ӧ����ɲ���ƽ�û�ѧ����ʽ��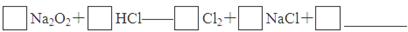
�÷�Ӧ________(��ܡ����ܡ�)����ʵ���ҿ�����ȡ������Cl2��������___________________________________________________________________________________________________________________________________(Ҫ����Ҫ��)��
��1��Cl2��4H����4I����O2=2I2��2H2O(��4I����O2��2H2O=2I2��4OH��)
��2������HCl������Cl2����ֹ��Ⱦ������ʹD�����ռ�����Ϊ����������
��3����Na2O2����ԭʱ��Ԫ�صĻ��ϼ�Ӧ�ý��ͣ������ܵõ�O2
��O2�п�����Na2O2�������е�H2O��Ӧ���ɵ�
��4�� 
���ܡ�ʵ����û�п�ֱ��ʹ�õĸ���HCl��������ʣ����������巴Ӧ�����������������к��д������Ȼ�������(����κ�һ�㼴�ɣ����������𰸾���)
���������������1��װ��B�е�ʪ��ĺ�ɫֽ����ɫ��˵�����ɵ���������ˮ���ڵ������¾���Ư���ԣ���Һ������Cl2����B�иķ�ʪ��ĵ���KI��ֽ��O2Ҳ��ʹ��ֽ��������Ӧ�����ӷ���ʽΪ��4H����4I����O2=2I2��2H2O(��4I����O2��2H2O=2I2��4OH��)��
��2��װ��Cʢ��NaOH��Һ��������HCl������Cl2����ֹ��Ⱦ������ʹD�����ռ�����Ϊ������������
��3���ٸ���������ԭ��Ӧԭ��������Na2O2����ԭʱ��Ԫ�صĻ��ϼ�Ӧ�ý��ͣ������ܵõ�O2��
�������к���H2O��Na2O2�������е�H2O��Ӧ���ɿ�����O2��
��4������Ԫ���غ㣬�����ﻹ��H2O�����ݻ��ϼ���������ƽ��ѧ����ʽ��Na2O2�����Ļ��ϼ۽���2�ۡ������������ϼ�����2�ۣ�����Na2O2��Cl2�Ļ�ѧ��������Ϊ1���ٵ�NaCl��H2O�Ļ�ѧ������Ϊ2���ܷ�����ʵ���ҿ�����ȡ������Cl2����Ҫ���Ƿ�Ӧ���Ƿ���ʵ�����д��ڣ���Ӧ�Ƿ������У������Ƿ�����Ϊʵ����û�п�ֱ��ʹ�õĸ���HCl��������ʣ����������巴Ӧ�����������������к��д������Ȼ������壬���Ը÷�Ӧ��������ʵ���ҿ�����ȡ������Cl2��
���㣺���⿼��ʵ�鷽���ķ��������ۡ�������Ʊ�����ѧ����ʽ����ƽ����д���Լ����õķ�����

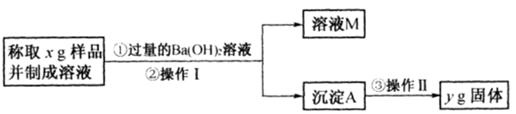
��16�֣�����ý(����ij������)�ǹ�ҵ�ϳɰ��Ĵ�����ijͬѧ������������ַ����о�����ý����ɡ�
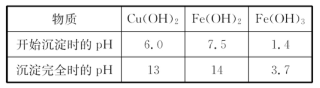
����һ�����������̲ⶨ����ý�ĺ�������ȷ������ɡ�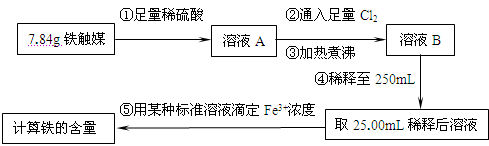
��1������ܺ������� (����������)ȡ25.00mLϡ�ͺ���Һ��
��2����Ϊͨ��Cl2������������ҺB���л����� ��Ӱ��ⶨ�����
��3����Ϊͨ��Cl2�����Ҽ�����в���֣�����ҺB���п��ܺ���Cl2�������ʵ�鷽������Cl2���������ʵ�鱨�档
��ѡ�Լ���0.1mol��L��1����KMnO4��Һ����ɫʯ����Һ��Ʒ��ϡ��Һ������-KI��Һ��0.1moL��L��1KSCN��Һ
| ʵ����� | ʵ����������� |
| | |
��������������ʵ�鷽���ⶨ����ý�ĺ�������ȷ������ɡ�
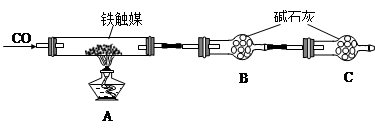
��4���������C���������� ��
��5����ȡ15.2g����ý��������ʵ�顣��ַ�Ӧ��á������B������11.0g���������ý�Ļ�ѧʽ�ɱ�ʾΪ ��(���ԭ��������C��12 O��16 Fe��56)
 (x��y)Cu��xCO2��(x��2y��z)H2O
(x��y)Cu��xCO2��(x��2y��z)H2O