��Ŀ����
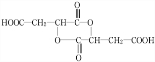
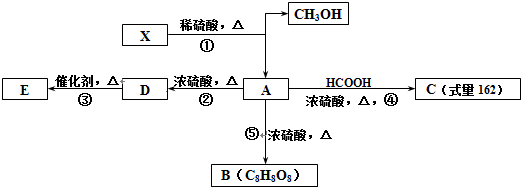
14��ʯ�ͻ�������Ҫԭ��CxHy���Ժϳɺܶ��л������������CxHy�ϳ�����E��J������ͼ����J�ķ���ʽΪC4H4O4����һ�ֻ�״�����
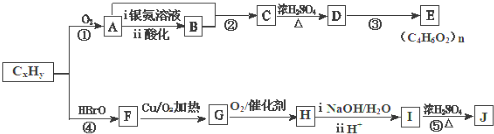
��֪�������з�Ӧ����R��R�����������

��J�ķ���ʽΪC4H4O4����һ�ֻ�״������
��1����CxHy��ͬϵ���У�����̼ԭ��һ����ƽ����̼ԭ�������ķ��ӵ�������2��3-����-2-��ϩ��
��2��H�ķ���ʽ��C2H3O2Br��
��3������˵����ȷ����be��
a��CxHy�ͱ�����ʹ��ˮ��ɫ��ԭ����ͬ b����Ӧ�ںͷ�Ӧ�ܵķ�Ӧ���;�Ϊ�ӳɷ�Ӧ
c��C����Na��NaOH��NaHCO3��Ӧ d��E��һ��ˮ���Ժܺõĸ߷��ӻ�����
e��J�����Ի���Ի����о���ˮ��
��4��K��J��ͬ���칹�壬��1mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬��д��һ�ַ�������K�Ľṹ��ʽCH2=C��COOH��2��HOOCCH=CHCOOH��
��5��д����Ӧ�ݵĻ�ѧ����ʽ
 ��
����6��D�ж���ͬ���칹�壬��D������ͬ�����ŵĻ���5�֣���˳���칹�壩�����к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽ��HCOOC��CH3��=CH2��
���� �������и�����ת����ϵ��J�ķ���ʽΪC4H4O4����һ�ֻ�״���������I��Ũ���������¼��ȵõ������Կ�����֪JΪ��������JΪ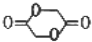 �������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E���ݴ˷������
�������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E���ݴ˷������
��� �⣺�������и�����ת����ϵ��J�ķ���ʽΪC4H4O4����һ�ֻ�״���������I��Ũ���������¼��ȵõ������Կ�����֪JΪ��������JΪ �������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
�������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
��1����C2H4��ͬϵ���У�����̼ԭ��һ����ƽ����̼ԭ�������ķ����ǽ���ϩ�е���ԭ�Ӷ�����̼�Ľṹ��C6H12����������2��3-����-2-��ϩ��
�ʴ�Ϊ��2��3-����-2-��ϩ�� ������������
��2��HΪBrCH2COOH��H�ķ���ʽ��C2H3O2Br��
�ʴ�Ϊ��C2H3O2Br��
��3��a��CxHy�ͱ�����ʹ��ˮ��ɫ��ԭ������ͬ��ǰ���Ǽӳɣ���������ȡ����a����
b����Ӧ�ںͷ�Ӧ�ܵķ�Ӧ���;�Ϊ�ӳɷ�Ӧ����b��ȷ��
c��C�����������ǻ������������������Ʒ�Ӧ���ǻ������Ʒ�Ӧ���������ǻ���������NaHCO3��Ӧ����c����
d��E������������ˮ���Խϲ��d����
e��J���������������Ի���Ի����о���ˮ�⣬��e��ȷ��
�ʴ�Ϊ��be��
��4��JΪ ��K��J��ͬ���칹�壬��1 mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬˵��K���������Ȼ������Է�������K�Ľṹ��ʽΪCH2=C��COOH��2����HOOCCH=CHCOOH��
��K��J��ͬ���칹�壬��1 mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬˵��K���������Ȼ������Է�������K�Ľṹ��ʽΪCH2=C��COOH��2����HOOCCH=CHCOOH��
�ʴ�Ϊ��CH2=C��COOH��2��HOOCCH=CHCOOH��
��5����Ӧ�ݵĻ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��DΪCH3COOCH=CH2��D�ж���ͬ���칹�壬��D������ͬ�����ŵĻ���CH2=CHCOOCH3��HCOOCH=CHCH3��˳�������֣���HCOOCH2CH=CH2��HCOOC��CH3��=CH2������5 �֣���˳���칹�壩�����к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽΪHCOOC��CH3��=CH2��
�ʴ�Ϊ��5��HCOOC��CH3��=CH2��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ����������������Ϣ����Ӧ�������������̼���仯�����ƶϼ��ɣ��ѵ���ͬ���칹�������жϣ���Ŀ�Ѷ��еȣ�

| A�� | ����ϩ�����߷��ӣ�����ʳƷ��װ�� | |
| B�� | ʯ�͵ĸ���õ����͡����͡�ú�� | |
| C�� | �����Ƶ�ˮ��Һ�׳�ˮ������������ľ�ķ���� | |
| D�� | ��ˮ����Ũ�����Ũ���ᰴ3��1��� |
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g/cm3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -1l6 |
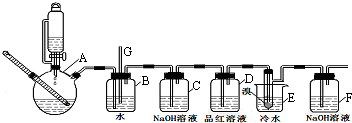
��1��ʵ�鿪ʼ֮ǰ��Ҫ�IJ����Ǽ��װ�õ������ԣ�
��2������A������Ϊ����ƿ��
��3��ʵ������У�������װ��B��ˮ�ص���G������������еIJ�����ֹͣ���ȣ����ձ�E�м�������ˮ��
��4��װ��D��Ʒ����Һ����������֤���������Ƿ�����
��5����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ���Ǽ���Һ��ӷ���
��6���жϸ��Ʊ���Ӧ�Ѿ��������������װ��E��С�Թ��ڵ�Һ���ɺ���ɫ��Ϊ��ɫ�����ѧ�����ַ�Ӧ����ʱ����ˮ�Ҵ��������������ֵ����ԭ�����и���Ӧ������Ӧ���ھ��ң�һ������ϩû�г�ַ�Ӧ���ݳ�����������������ɣ���
| A�� | ��֪���淴Ӧ��2SO2+O2?2SO3����O2������������SO3����������֮��Ϊ1��2ʱ�������ÿ��淴Ӧ�ﵽ��ѧƽ��״̬ | |
| B�� | ��Zn��Cu��ϡ������ɵ�ԭ��أ�����ΪZn��������ԭ��Ӧ���缫��ӦʽΪ��Zn-2e-=Zn2+ | |
| C�� | ��ҵ�Ͻ���Mg��Al�����õ�����ڵ��Ȼ����Ƶõ� | |
| D�� | �ױ���һ�ȴ�����4�֣���ױ�������H2�����ӳɷ�Ӧ�����õ����л������һ�ȴ���Ҳֻ��4�� |
| ��������� | ���� |
| ���������Ũ�ȵ�AgNO3��Һ��NaCl��Һ��ϵõ���ҺW�����ˣ��õ���ҺX�Ͱ�ɫ����Y | |
| ������ҺX�еμӼ��α���Na2S��Һ | ���ֻ��� |
| ��ȡ������ɫ����Y���μӼ��α���Na2S��Һ | ������Ϊ��ɫ |
| ����ȡ������ɫ����Y���μӼ���Ũ��ˮ | �������ܽ� |
 Ag+��aq��+Cl-��aq����
Ag+��aq��+Cl-��aq������2���ɲ����Ļ��ǿ��Ʋ⣬��ҺX�г��˺���Na+��NO3-�������е�������Ag+��Cl-��
��3����˵��������г�����ڵ����ӷ���ʽΪ2AgCl��s��+S2-�TAg2S��s��+2Cl-������ת������Ҫԭ���Ǹ��¶��£�Ag2S��AgCl�ܽ�ȸ�С��
��4����֪��Ag++2NH3•H2O Ag��NH3��2++2H2O����ƽ���ƶ�ԭ�����Ͳ�����м���Ũ��ˮ�������ܽ��ԭ��AgCl��g��
 Ag+��aq��+Cl-��aq�����������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ⣮
Ag+��aq��+Cl-��aq�����������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ⣮��5�����������Ϣ����������Ԥ�ⲻ��ȷ����BC��
A���ڲ����֮�����μ�Ũ���������AgCl��������
B���ɲ���������Ʋ⣺ʵ���ҿ��ð�ˮϴ��������Ӧ����Թ�
C�����ڰ�ɫ����Y�еμ�NaOH��Һ������Ҳ���ܽ⣮
| A�� | 100mL��0.01mol/L��CH3COOH��Һ��10mL��0.1mol/L��CH3COOH��Һ��H+��Ŀ | |
| B�� | ������pH=4��KHSO4��Һ��CH3COOK��Һ����ˮ�������OH-����Ũ�� | |
| C�� | ��ˮ��100��ʱ��pH��25��ʱ��pH | |
| D�� | 100mL��0.01mol/L��CH3COOH��Һ��10mL��0.1mol/L��CH3COOH��Һ��H+Ũ�� |
| A�� | 2 | B�� | 3 | C�� | 4 | D�� | 5 |
 ��ͼ�ǿ��淴ӦN2��g��+3H2��g���T2NH3��g�� �ڷ�Ӧ�����еķ�Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ���ߣ�������������ȷ���ǣ�������
��ͼ�ǿ��淴ӦN2��g��+3H2��g���T2NH3��g�� �ڷ�Ӧ�����еķ�Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ���ߣ�������������ȷ���ǣ�������| A�� | t1ʱ��v����v�� | B�� | t2ʱ����Ӧ������ | ||
| C�� | t2-t3����Ӧֹͣ | D�� | t2-t3�������ʵ�Ũ�Ȳ��ٷ����仯 |
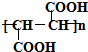
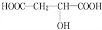 +HCOOH$?_{��}^{Ũ����}$
+HCOOH$?_{��}^{Ũ����}$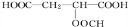 +H2O
+H2O