��Ŀ����
����Ŀ���ϳɹ�̽���һ��·�����£�


��RCOOH+CH![]() CH��RCOOCH=CH2
CH��RCOOCH=CH2
�ش���������
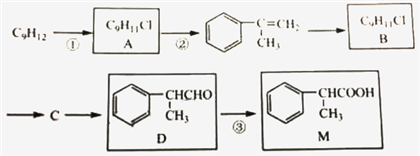
��1����̽������������ŵ�������______��
��2��D+G����̽��Ļ�ѧ����ʽΪ______���÷�Ӧ�ķ�Ӧ������______��
��3��H��C��ͬ���칹�壬H�����������ʻ����������ܷ���ˮ�ⷴӦ��������Ӧ������ʹ��ˮ��ɫ�������ڷ����廯�����H�Ľṹ��______�֡����к˴Ź�������Ϊ5��壬�ҷ������Ϊ1��1��2��2��2�Ľṹ��ʽΪ______��
��4����������֪ʶ����������Ϣ��д����CH3CH2OHΪԭ���Ʊ�CH3CH2CH2COOC2H5�ĺϳ�·������ͼ(���Լ���ѡ)��
���ϳ�·������ͼʾ�����£�![]() ��
��
______
���𰸡� ̼̼˫�������� 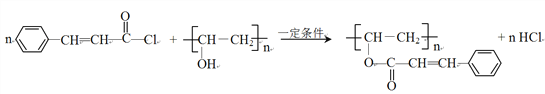 ȡ����Ӧ 5
ȡ����Ӧ 5 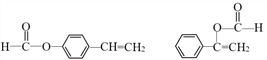 CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]() CH3CH=CHCHO
CH3CH=CHCHO![]() CH3CH2CH2CH2OH
CH3CH2CH2CH2OH![]() CH3CH2CH2COOH
CH3CH2CH2COOH![]() CH3CH2CH2COOC2H5
CH3CH2CH2COOC2H5
�����������ݸ����ʵ�ת����ϵ��A������Ϣ���еķ�Ӧ����B��B����������Ӧ����C��C����ȡ����Ӧ����D������D�Ľṹ��ʽ��֪��CΪ![]() ��BΪ
��BΪ![]() ��AΪ
��AΪ![]() ��������Ϣ�����E�ķ���ʽ��֪������XΪCH3COOH��EΪCH3COOCH=CH2��E�����Ӿ۷�Ӧ����FΪ
��������Ϣ�����E�ķ���ʽ��֪������XΪCH3COOH��EΪCH3COOCH=CH2��E�����Ӿ۷�Ӧ����FΪ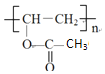 ��F����ȡ������G��G��D����ȡ����Ӧ���ɹ�̽�Ϊ
��F����ȡ������G��G��D����ȡ����Ӧ���ɹ�̽�Ϊ ��
��
��1����̽�Ϊ �������������ŵ�������̼̼˫�����������ʴ�Ϊ��̼̼˫����������
�������������ŵ�������̼̼˫�����������ʴ�Ϊ��̼̼˫����������
��2����������ķ�����֪��G��D����ȡ����Ӧ���ɹ�̽�Ϊ ����Ӧ�Ļ�ѧ����ʽΪ
����Ӧ�Ļ�ѧ����ʽΪ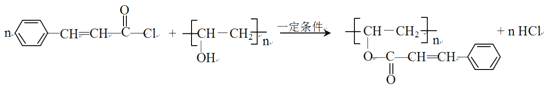 ���ʴ�Ϊ��
���ʴ�Ϊ��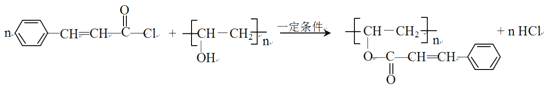 ��ȡ����Ӧ��
��ȡ����Ӧ��
��3��CΪ![]() ��H��C��ͬ���칹�壬T�����������ʻ����������ܷ���ˮ�ⷴӦ��������Ӧ��˵���м���ij��������ʹ��ˮ��ɫ��˵����̼̼˫���������ڷ����廯���˵���б���������������ĽṹΪ����������HCOOCH=CH-����HCOOC��=CH2��-������������������ΪHCOO-��-CH=CH2����3�ֽṹ�����Թ���5�֣����к˴Ź�������Ϊ5��壬�ҷ������Ϊ1��1��2��2��2�Ľṹ��ʽΪ
��H��C��ͬ���칹�壬T�����������ʻ����������ܷ���ˮ�ⷴӦ��������Ӧ��˵���м���ij��������ʹ��ˮ��ɫ��˵����̼̼˫���������ڷ����廯���˵���б���������������ĽṹΪ����������HCOOCH=CH-����HCOOC��=CH2��-������������������ΪHCOO-��-CH=CH2����3�ֽṹ�����Թ���5�֣����к˴Ź�������Ϊ5��壬�ҷ������Ϊ1��1��2��2��2�Ľṹ��ʽΪ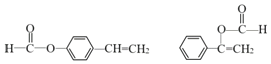 ���ʴ�Ϊ��5��
���ʴ�Ϊ��5��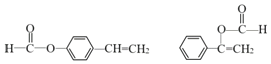 ��
��
��4����CH3CH2OHΪԭ���Ʊ�CH3CH2CH2COOH����CH3CH2OH������CH3CHO��CH3CHO��ϡ�������Ƶ�����������CH3CH=CHCHO���������ӳɵ�CH3CH2CH2CH2OH���ٽ�CH3CH2CH2CH2OH������CH3CH2CH2COOH����������Ҵ��������ɣ��ϳ�·��ΪCH3CH2OH ![]() CH3CHO
CH3CHO![]() CH3CH=CHCHO
CH3CH=CHCHO![]() CH3CH2CH2CH2OH
CH3CH2CH2CH2OH ![]() CH3CH2CH2COOH
CH3CH2CH2COOH![]() CH3CH2CH2COOC2H5���ʴ�Ϊ��CH3CH2OH
CH3CH2CH2COOC2H5���ʴ�Ϊ��CH3CH2OH ![]() CH3CHO
CH3CHO![]() CH3CH=CHCHO
CH3CH=CHCHO![]() CH3CH2CH2CH2OH
CH3CH2CH2CH2OH![]() CH3CH2CH2COOH
CH3CH2CH2COOH![]() CH3CH2CH2COOC2H5��
CH3CH2CH2COOC2H5��