题目内容
(16分)某班学生在老师指导下探究氮的化合物的某些性质。
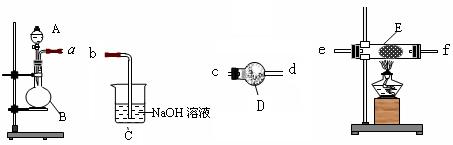
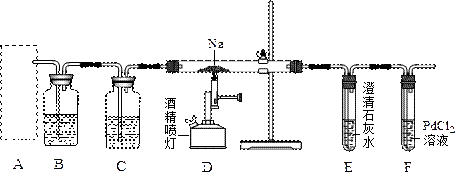
(1)同学甲在实验室利用下列装置(后面有图)制取氨气和氧气的混合气体,并完成氨的催化氧化。
A中加入浓氨水,D中加入碱石灰,E内放置催化剂(铂石棉),请回答:
①仪器B的名称:__________。B内只需加 入一种固体试剂,该试剂的名称为_________,B中能产生氨气和氧气混合气体的原
入一种固体试剂,该试剂的名称为_________,B中能产生氨气和氧气混合气体的原 因(结合化学方程式回答)_ __。
因(结合化学方程式回答)_ __。
②按气流方向连接各仪器 (填接口字母)
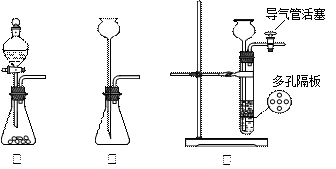
(2)同学乙拟用甲同学得到的混合气体X(NO及过量的NH3),验证NO能被氨气还原并测算其转化率(忽略装置内空气的影响)。装置如下:
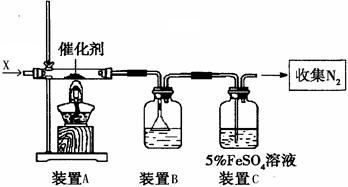
①装置C的作用可能是 ____________。
②若进入装置A的NO共268.8mL(已折算为标准状况,下同),氨气过量,最后收集到标准状况下190.4 mL N2,则NO的转化率为 。
(3)N2O3是一种新型硝化剂。一定温度下,在恒容密闭容器中N2O3可发生下列反应:2N2O3+O2 4NO2(g);△H>0,下表为反应在某温度下的部分实验数据:
4NO2(g);△H>0,下表为反应在某温度下的部分实验数据:
计算在t=500s时,NO2的反应速率为 。
(1)同学甲在实验室利用下列装置(后面有图)制取氨气和氧气的混合气体,并完成氨的催化氧化。
A中加入浓氨水,D中加入碱石灰,E内放置催化剂(铂石棉),请回答:
①仪器B的名称:__________。B内只需加
 入一种固体试剂,该试剂的名称为_________,B中能产生氨气和氧气混合气体的原
入一种固体试剂,该试剂的名称为_________,B中能产生氨气和氧气混合气体的原 因(结合化学方程式回答)_ __。
因(结合化学方程式回答)_ __。②按气流方向连接各仪器 (填接口字母)

(2)同学乙拟用甲同学得到的混合气体X(NO及过量的NH3),验证NO能被氨气还原并测算其转化率(忽略装置内空气的影响)。装置如下:

①装置C的作用可能是 ____________。
②若进入装置A的NO共268.8mL(已折算为标准状况,下同),氨气过量,最后收集到标准状况下190.4 mL N2,则NO的转化率为 。
(3)N2O3是一种新型硝化剂。一定温度下,在恒容密闭容器中N2O3可发生下列反应:2N2O3+O2
 4NO2(g);△H>0,下表为反应在某温度下的部分实验数据:
4NO2(g);△H>0,下表为反应在某温度下的部分实验数据:| t/s | 0 | 500 | 1000 |
| c(N2O3)/mol·L-1 | 5.00 | 3.52 | 2.48 |
计算在t=500s时,NO2的反应速率为 。
(1)①圆底烧瓶 过氧化钠
2Na2O2+2NH3·H2O=4NaOH+O2↑+2NH3↑过氧化钠与氨水反应消耗水,增大了氨水浓度;过氧化钠与水反应生成氧气,反应放热减小了氨气的溶解度;过氧化钠与水反应生成NaOH,电离出OH—,使NH3·H2O的电离平衡逆向移动(3分) ②a c d e f b或a c d f e b
(2)①吸收未反应的NO ②85%(3分)(3)0.00592mol·L-1·s-1(不写单位不得分)
2Na2O2+2NH3·H2O=4NaOH+O2↑+2NH3↑过氧化钠与氨水反应消耗水,增大了氨水浓度;过氧化钠与水反应生成氧气,反应放热减小了氨气的溶解度;过氧化钠与水反应生成NaOH,电离出OH—,使NH3·H2O的电离平衡逆向移动(3分) ②a c d e f b或a c d f e b
(2)①吸收未反应的NO ②85%(3分)(3)0.00592mol·L-1·s-1(不写单位不得分)
略

练习册系列答案
 学而优衔接教材南京大学出版社系列答案
学而优衔接教材南京大学出版社系列答案 小学课堂作业系列答案
小学课堂作业系列答案 金博士一点全通系列答案
金博士一点全通系列答案
相关题目

 Ⅰ.查阅资料:①Cu2O属于碱性氧化物; ②在空气中灼烧Cu2O生成CuO;③Cu2O在酸性条件下能发生下列反应:Cu2O+2H+=Cu+Cu2++H2O。
Ⅰ.查阅资料:①Cu2O属于碱性氧化物; ②在空气中灼烧Cu2O生成CuO;③Cu2O在酸性条件下能发生下列反应:Cu2O+2H+=Cu+Cu2++H2O。 验方案:
验方案:

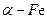 粉具有超强的磁性能,可用作高密度磁记录的介质以及高效催化剂等。在不同温度下,
粉具有超强的磁性能,可用作高密度磁记录的介质以及高效催化剂等。在不同温度下,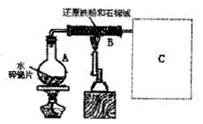
 葡萄糖(C6H12O6)
葡萄糖(C6H12O6)