ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ Β―ι “÷Τ±Η±ΫΦΉ¥ΦΚΆ±ΫΦΉΥαΒΡΜ·―ß‘≠άμ «2C6H5CHOΘΪNaOH![]() C6H5CH2OHΘΪC6H5COONaΘ§C6H5COONaΘΪHClΓζC6H5COOHΘΪNaClΓΘ“―÷Σ±ΫΦΉ»©“Ή±ΜΩ’Τχ―θΜ·ΘΜ±ΫΦΉ¥ΦΒΡΖ–ΒψΈΣ205.3 ΓφΘ§ΈΔ»ή”ΎΥ°Θ§“Ή»ή”Ύ““Ο―ΘΜ±ΫΦΉΥαΒΡ»έΒψΈΣ121.7 ΓφΘ§Ζ–ΒψΈΣ249 ΓφΘ§ΈΔ»ή”ΎΥ°Θ§“Ή»ή”Ύ““Ο―ΘΜ““Ο―ΒΡΖ–ΒψΈΣ34.8 ΓφΘ§Ρ―»ή”ΎΥ°ΓΘ÷Τ±Η±ΫΦΉ¥ΦΚΆ±ΫΦΉΥαΒΡ÷ς“ΣΙΐ≥Χ»γΆΦΥυ ΨΘ§ΗυΨί“‘…œ–≈œΔ≈–ΕœΘ§œ¬Ν–ΥΒΖ®¥μΈσΒΡ «
C6H5CH2OHΘΪC6H5COONaΘ§C6H5COONaΘΪHClΓζC6H5COOHΘΪNaClΓΘ“―÷Σ±ΫΦΉ»©“Ή±ΜΩ’Τχ―θΜ·ΘΜ±ΫΦΉ¥ΦΒΡΖ–ΒψΈΣ205.3 ΓφΘ§ΈΔ»ή”ΎΥ°Θ§“Ή»ή”Ύ““Ο―ΘΜ±ΫΦΉΥαΒΡ»έΒψΈΣ121.7 ΓφΘ§Ζ–ΒψΈΣ249 ΓφΘ§ΈΔ»ή”ΎΥ°Θ§“Ή»ή”Ύ““Ο―ΘΜ““Ο―ΒΡΖ–ΒψΈΣ34.8 ΓφΘ§Ρ―»ή”ΎΥ°ΓΘ÷Τ±Η±ΫΦΉ¥ΦΚΆ±ΫΦΉΥαΒΡ÷ς“ΣΙΐ≥Χ»γΆΦΥυ ΨΘ§ΗυΨί“‘…œ–≈œΔ≈–ΕœΘ§œ¬Ν–ΥΒΖ®¥μΈσΒΡ «
A.≤ΌΉςΔώ «ίΆ»ΓΖ÷“Κ
B.≤ΌΉςΔρ’τΝσΒΟΒΫΒΡ≤ζΤΖΦΉ «±ΫΦΉ¥Φ
C.≤ΌΉςΔσΙΐ¬ΥΒΟΒΫΒΡ≤ζΤΖ““ «±ΫΦΉΥαΡΤ
D.““Ο―»ή“Κ÷–Υυ»ήΫβΒΡ÷ς“Σ≥…Ζ÷ «±ΫΦΉ¥Φ
ΓΨ¥πΑΗΓΩC
ΓΨΫβΈωΓΩ
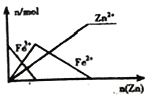
AΓΔ”…Νς≥ΧΩ…÷ΣΘ§±ΫΦΉ»©”κKOHΖ¥”Π…ζ≥…±ΫΦΉ¥ΦΓΔ±ΫΦΉΥαΦΊΘ§»ΜΚσΦ”Υ°ΓΔ““Ο―ίΆ»Γ±ΫΦΉ¥ΦΘ§‘ρ““Ο―»ή“Κ÷–Κ§±ΫΦΉ¥ΦΘ§Υυ“‘≤ΌΉςΔώ «ίΆ»ΓΖ÷“ΚΘ§Ι A’ΐ»ΖΘΜ
BΓΔ”…Νς≥ΧΩ…÷ΣΘ§≤ΌΉςΔρ «Ζ÷άκ““Ο―ΚΆ±ΫΦΉ¥ΦΘ§ΗυΨί““Ο―ΓΔ±ΫΦΉ¥ΦΒΡΖ–Βψ≤ΜΆ§Θ§”Ο’τΝσΖ®Ζ÷άκ““Ο―ΚΆ±ΫΦΉ¥ΦΘ§Ι B’ΐ»ΖΘΜ
CΓΔΥ°»ή“Κ÷–Κ§±ΫΦΉΥαΦΊΘ§Φ”―ΈΥαΖΔ…ζ«ΩΥα÷Τ»Γ»θΥαΒΡΖ¥”ΠΘ§…ζ≥…±ΫΦΉΥαΘ§±ΫΦΉΥαΒΡ»ήΫβΕ»–ΓΘ§…ζ≥…±ΫΦΉΥα≥ΝΒμΘ§Ά®Ιΐ≤ΌΉςΔσΙΐ¬ΥΒΟΒΫΒΡ≤ζΤΖ““ «±ΫΦΉΥαΘ§Ι C¥μΈσΘΜ
DΓΔ”…Νς≥ΧΩ…÷ΣΘ§ΑΉ…ΪΚΐΉ¥Έο÷–Κ§”–±ΫΦΉ¥ΦΚΆ±ΫΦΉΥαΦΊΘ§±ΫΦΉ¥Φ“Ή»ή”Ύ““Ο―Θ§±ΫΦΉΥαΦΊ «―ΈΘ§“Ή»ή”ΎΥ°Θ§Υυ“‘““Ο―»ή“Κ÷–Υυ»ήΫβΒΡ÷ς“Σ≥…Ζ÷ «±ΫΦΉ¥ΦΘ§Ι D’ΐ»ΖΓΘ
―ΓCΓΘ

 ’ψ¥σ”≈―ß–Γ―ßΡξΦΕœΈΫ”ΫίΨΕ’ψΫ≠¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗ
’ψ¥σ”≈―ß–Γ―ßΡξΦΕœΈΫ”ΫίΨΕ’ψΫ≠¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ Β―ι “¥”Κ§ΒβΖœ“Κ![]() Κ§”–
Κ§”–![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() Β»
Β»![]() ÷–ΜΊ ’ΒβΘ§≤ΌΉςΙΐ≥Χ»γœ¬ΘΚ
÷–ΜΊ ’ΒβΘ§≤ΌΉςΙΐ≥Χ»γœ¬ΘΚ
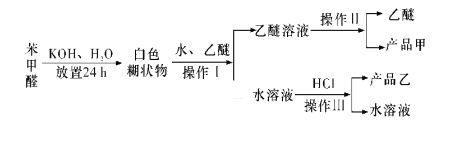
![]() œρΖœ“Κ÷–Φ”»κ
œρΖœ“Κ÷–Φ”»κ![]() »ή“ΚΘ§ΖΔ…ζΓΑΜΙ‘≠Γ±Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ ______ΓΘ
»ή“ΚΘ§ΖΔ…ζΓΑΜΙ‘≠Γ±Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ ______ΓΘ
![]() ΓΑ―θΜ·Γ±≤ΌΉς‘Ύ»ΐΨ±…’ΤΩ÷–Ϋχ––Θ§ΫΪ»ή“Κ”Ο―ΈΥαΒς÷ΝpH‘ΦΈΣ2Θ§ΜΚ¬ΐΆ®»κ
ΓΑ―θΜ·Γ±≤ΌΉς‘Ύ»ΐΨ±…’ΤΩ÷–Ϋχ––Θ§ΫΪ»ή“Κ”Ο―ΈΥαΒς÷ΝpH‘ΦΈΣ2Θ§ΜΚ¬ΐΆ®»κ![]() ΓΘ Β―ι”ΟΥ°‘ΓΩΊ÷Τ‘Ύ
ΓΘ Β―ι”ΟΥ°‘ΓΩΊ÷Τ‘Ύ![]() Ήσ”“ΒΡΫœΒΆΈ¬Ε»œ¬Ϋχ––ΒΡ‘≠“ρ « ______ΓΘ
Ήσ”“ΒΡΫœΒΆΈ¬Ε»œ¬Ϋχ––ΒΡ‘≠“ρ « ______ΓΘ
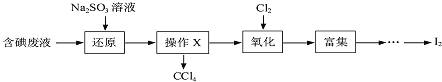
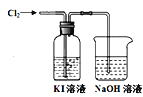
![]() Ρ≥―–ΨΩ–ΓΉι”ΟΆΦΉΑ÷ΟΕ‘
Ρ≥―–ΨΩ–ΓΉι”ΟΆΦΉΑ÷ΟΕ‘![]() ”κKI»ή“ΚΒΡΖ¥”ΠΫχ––ΧΫΨΩΘ§ΖΔœ÷Ά®»κ
”κKI»ή“ΚΒΡΖ¥”ΠΫχ––ΧΫΨΩΘ§ΖΔœ÷Ά®»κ![]() “ΜΕΈ ±ΦδΚσΘ§KI»ή“Κ±δΈΣΜΤ…ΪΘ§ΦΧ–χΆ®»κ
“ΜΕΈ ±ΦδΚσΘ§KI»ή“Κ±δΈΣΜΤ…ΪΘ§ΦΧ–χΆ®»κ![]() Θ§‘ρ»ή“ΚΜΤ…Ϊ±δ«≥Θ§ΉνΚσ±δΈΣΈό…ΪΘ°―–ΨΩ–ΓΉιΕ‘ΥυΒΟΈό…Ϊ»ή“Κ÷–Ββ‘ΣΥΊΒΡ¥φ‘Ύ–ΈΧ§Χα≥ωΝΥ“‘œ¬ΦΌ…ηΘΚ
Θ§‘ρ»ή“ΚΜΤ…Ϊ±δ«≥Θ§ΉνΚσ±δΈΣΈό…ΪΘ°―–ΨΩ–ΓΉιΕ‘ΥυΒΟΈό…Ϊ»ή“Κ÷–Ββ‘ΣΥΊΒΡ¥φ‘Ύ–ΈΧ§Χα≥ωΝΥ“‘œ¬ΦΌ…ηΘΚ
ΦΌ…η“ΜΘΚΟΜ”–![]() –ΈΧ§ΘΜΦΌ…ηΕΰΘΚΟΜ”–
–ΈΧ§ΘΜΦΌ…ηΕΰΘΚΟΜ”–![]() –ΈΧ§ΘΜΦΌ…η»ΐΘΚ”–
–ΈΧ§ΘΜΦΌ…η»ΐΘΚ”–![]() –ΈΧ§ΓΘ
–ΈΧ§ΓΘ
![]() «κ…ηΦΤ Β―ι÷ΛΟςΦΌ…η“Μ≥…ΝΔ
«κ…ηΦΤ Β―ι÷ΛΟςΦΌ…η“Μ≥…ΝΔ![]() ‘ΦΝΉ‘―Γ
‘ΦΝΉ‘―Γ![]() ΓΘ
ΓΘ
Β―ι≤ΌΉς | ‘ΛΤΎœ÷œσ | Ϋα¬έ |
_______ | _______ | ΦΌ…η“Μ≥…ΝΔ |
![]() »τΦΌ…η»ΐ≥…ΝΔΘ§«κ–¥≥ω…ζ≥…
»τΦΌ…η»ΐ≥…ΝΔΘ§«κ–¥≥ω…ζ≥…![]() ΒΡάκΉ”ΖΫ≥Χ Ϋ ______ΓΘ
ΒΡάκΉ”ΖΫ≥Χ Ϋ ______ΓΘ
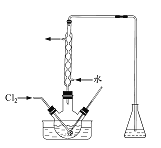
![]() ΗΟ―–ΨΩ–ΓΉιΜΙΫχ––ΝΥΕ‘Φ”Ββ―Έ÷–
ΗΟ―–ΨΩ–ΓΉιΜΙΫχ––ΝΥΕ‘Φ”Ββ―Έ÷–![]() Κ§ΝΩ≤βΕ®ΒΡ»γœ¬ Β―ιΘΚ
Κ§ΝΩ≤βΕ®ΒΡ»γœ¬ Β―ιΘΚ
![]() ΉΦ»Ζ≥Τ»ΓΦ”Ββ―Έmg”Ύ…’±≠÷–Θ§Φ”»κ ΝΩ’τΝσΥ°ΚΆΙΐΝΩΒΡKIΘ§‘ΌΒΈ»κ ΝΩΒΡœΓΝρΥαΘ§≥δΖ÷Ζ¥”ΠΘ§ΫΪΥυΒΟΜλΚœ“Κ≈δ≥…
ΉΦ»Ζ≥Τ»ΓΦ”Ββ―Έmg”Ύ…’±≠÷–Θ§Φ”»κ ΝΩ’τΝσΥ°ΚΆΙΐΝΩΒΡKIΘ§‘ΌΒΈ»κ ΝΩΒΡœΓΝρΥαΘ§≥δΖ÷Ζ¥”ΠΘ§ΫΪΥυΒΟΜλΚœ“Κ≈δ≥…![]() ¥ΐ≤β»ή“ΚΘ°“Τ»Γ
¥ΐ≤β»ή“ΚΘ°“Τ»Γ![]() ¥ΐ≤β»ή“Κ”ΎΉΕ–ΈΤΩ÷–Θ§Φ”ΦΗΒΈΒμΖέ ‘“ΚΘ§”Οc
¥ΐ≤β»ή“Κ”ΎΉΕ–ΈΤΩ÷–Θ§Φ”ΦΗΒΈΒμΖέ ‘“ΚΘ§”Οc![]()
![]() ±ξΉΦ“ΚΒΈΕ®÷Ν÷’ΒψΘ§÷ΊΗ¥3¥ΈΘ§≤βΒΟΤΫΨυ÷ΒΈΣVmLΓΘ
±ξΉΦ“ΚΒΈΕ®÷Ν÷’ΒψΘ§÷ΊΗ¥3¥ΈΘ§≤βΒΟΤΫΨυ÷ΒΈΣVmLΓΘ
“―÷ΣΘΚ![]() Θ§
Θ§![]() ΓΘ≤βΕ® ±Θ§≈–Εœ¥οΒΫΒΈΕ®÷’ΒψΒΡœ÷œσΈΣ ______ Θ§”…≤βΕ® ΐΨίΩ…«σΒΟΗΟ―υΤΖ÷–Κ§
ΓΘ≤βΕ® ±Θ§≈–Εœ¥οΒΫΒΈΕ®÷’ΒψΒΡœ÷œσΈΣ ______ Θ§”…≤βΕ® ΐΨίΩ…«σΒΟΗΟ―υΤΖ÷–Κ§![]() ΒΡ÷ ΝΩΖ÷ ΐΈΣ ______
ΒΡ÷ ΝΩΖ÷ ΐΈΣ ______ ![]() ”ΟΚ§mΓΔcΓΔVΒΡ¥ζ ΐ Ϋ±μ ΨΘ§
”ΟΚ§mΓΔcΓΔVΒΡ¥ζ ΐ Ϋ±μ ΨΘ§![]()
![]() ΓΘ
ΓΘ
![]() ‘ΎΒΈΕ®≤ΌΉς’ΐ»ΖΈόΈσΒΡ«ιΩωœ¬Θ§”Ο¥Υ÷÷≤βΕ®ΖΫΖ®≤βΒΟΒΡΫαΙϊΆυΆυΤΪΗΏΘ§Τδ‘≠“ρ « ήΩ’ΤχΒΡ”ΑœλΘ§«κ”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ≤ζ…ζ’β“Μ”ΑœλΒΡ‘≠“ρ ______ΓΘ
‘ΎΒΈΕ®≤ΌΉς’ΐ»ΖΈόΈσΒΡ«ιΩωœ¬Θ§”Ο¥Υ÷÷≤βΕ®ΖΫΖ®≤βΒΟΒΡΫαΙϊΆυΆυΤΪΗΏΘ§Τδ‘≠“ρ « ήΩ’ΤχΒΡ”ΑœλΘ§«κ”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ≤ζ…ζ’β“Μ”ΑœλΒΡ‘≠“ρ ______ΓΘ
ΓΨΧβΡΩΓΩΡ≥Έ¬Ε»œ¬Θ§0.200 molL-1ΒΡHA»ή“Κ”κ0.200 molL-1ΒΡNaOH»ή“ΚΒ»ΧεΜΐΜλΚœΚσΘ§ΥυΒΟ»ή“Κ÷–≤ΩΖ÷ΈΔΝΘΉιΖ÷ΦΑ≈®Ε»»γœ¬±μΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
ΈΔΝΘ | X | Y | Na+ | A- |
≈®Ε»/Θ®molΓΛL-1Θ© | 8.00 | 2.50 | 0.100 | 9.92 |
A. 0.1molΓΛL-1HA»ή“ΚΒΡpH=1 B. ΗΟΈ¬Ε»œ¬Kw=1.0![]() 10-14
10-14
C. ΈΔΝΘX±μ ΨOH-Θ§Y±μ ΨH+ D. ΜλΚœ»ή“Κ÷–ΘΚnΘ®A-Θ©+nΘ®XΘ©=nΘ®Na+Θ©