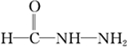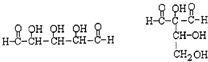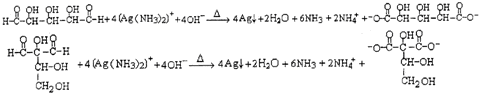��Ŀ����
��12�֣�
��1����״���£����ԼΪ11.2 L��NH3��Լ���� �����ӡ����� �����ӡ�
��2��ͬ��ͬѹ�£�ͬ�����İ������������壨H2S���������Ϊ ��ͬ��ͬѹ�£���������ԭ������ȣ����ǵ������Ϊ ��
��3����ij�¶�ʱ��һ������Ԫ��A���⻯��AH3��һ������ܱ������п���ȫ�ֽ��������̬���ʣ���ʱ�ܱ���������������ܵ����ʵ���������75%����A���ʵ�һ����������_____��Aԭ�ӣ�AH3�ֽⷴӦ�Ļ�ѧ����ʽΪ___________________��
��1��3.01��1023��2�֣� 3.01��1024��2�֣�
��2��2�U1��2�֣� 2�U3��2�֣�
��3��4��2�֣� 4AH3 = A4+6H2��2�֣�
����

��ϰ��ϵ�д�
�����Ŀ
 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��