��Ŀ����
����Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����

����д���пհף�
��1����֪0.5mol�����0.5molˮ������t�棬p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�
��2���ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪����������Ӧ�û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
��3��������ϳɰ�����ת����Ϊ75%ʱ����5.60��107L����Ϊԭ���ܹ��ϳ�
��4����֪���صĽṹ��ʽΪ ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��
 ����
����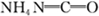
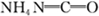 ��
��

����д���пհף�
��1����֪0.5mol�����0.5molˮ������t�棬p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�
CH4��g��+H2O��g��=CO��g��+3H2��g����H=+2aKJ/mol
CH4��g��+H2O��g��=CO��g��+3H2��g����H=+2aKJ/mol
����2���ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪����������Ӧ�û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
���������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ���������
���������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ���������
��3��������ϳɰ�����ת����Ϊ75%ʱ����5.60��107L����Ϊԭ���ܹ��ϳ�
1.12��108
1.12��108
L����������������ڱ�״���²ⶨ������4����֪���صĽṹ��ʽΪ
 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����


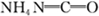
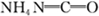
��������1��Ҫע�⻯ѧ���������H����ֵ��һ�£�ͬʱ��Ҫע�ⷴӦ����������״̬��
��2����������������ɼ���������Ũ�ȣ���ѧƽ�������ƶ������䵪��������������������Ũ�ȣ���������Ӧ���ʣ�
��3�����ݼ������������������ϳɰ��Ļ�ѧ����ʽ���ҳ������백�Ĺ�ϵʽ�����м��㣮
CH4+H2O�TCO+3H2��
CO+H2O�TCO2+H2��
N2+3H2�T2NH3����ϳɰ������ΪX���ʿɵù�ϵʽ��
CH4������
NH3
��4���������ط��ӽṹд���ṹ��ʽ����̼��˫����ͬ���칹���Ƿ���ʽ��������ͬ���ṹ��ͬ�����ʣ�
��2����������������ɼ���������Ũ�ȣ���ѧƽ�������ƶ������䵪��������������������Ũ�ȣ���������Ӧ���ʣ�
��3�����ݼ������������������ϳɰ��Ļ�ѧ����ʽ���ҳ������백�Ĺ�ϵʽ�����м��㣮
CH4+H2O�TCO+3H2��
CO+H2O�TCO2+H2��
N2+3H2�T2NH3����ϳɰ������ΪX���ʿɵù�ϵʽ��
CH4������
| 8 |
| 3 |
��4���������ط��ӽṹд���ṹ��ʽ����̼��˫����ͬ���칹���Ƿ���ʽ��������ͬ���ṹ��ͬ�����ʣ�
����⣺��1��0.5mol�����0.5molˮ������t�棬p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ������1mol�����1molˮ������ȫ��Ӧ����������һ����̼��������2aKJ��������Ӧ���Ȼ�ѧ����ʽΪ��CH4��g��+H2O��g��=CO��g��+3H2��g����H=+2aKJ/mol��
�ʴ�Ϊ��CH4��g��+H2O��g��=CO��g��+3H2��g����H=+2aKJ/mol��
��2���ںϳɰ���ʵ�����������У���ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪�������������������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ��������У�
�ʴ�Ϊ�����������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ��������У�
��3�����ݼ������������������ϳɰ��Ļ�ѧ����ʽ��CH4+H2O�TCO+3H2��CO+H2O�TCO2+H2��N2+3H2�T2NH3���ʿɵù�ϵʽ��
CH4������
NH3
1
5.60��107L��75% X
X=1.12��108L��
�ʴ�Ϊ��1.12��108��
��4�����صĽṹ��ʽΪ�� ������˫����������ͬ���칹������ʣ����Ըı�̼��˫�������ӵ�ԭ���Ż�ԭ��д����ͬ���칹��Ϊ��
������˫����������ͬ���칹������ʣ����Ըı�̼��˫�������ӵ�ԭ���Ż�ԭ��д����ͬ���칹��Ϊ�� ��
��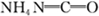 ��
��
�ʴ�Ϊ�� ��
��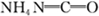 ��
��
�ʴ�Ϊ��CH4��g��+H2O��g��=CO��g��+3H2��g����H=+2aKJ/mol��
��2���ںϳɰ���ʵ�����������У���ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪�������������������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ��������У�
�ʴ�Ϊ�����������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ��������У�
��3�����ݼ������������������ϳɰ��Ļ�ѧ����ʽ��CH4+H2O�TCO+3H2��CO+H2O�TCO2+H2��N2+3H2�T2NH3���ʿɵù�ϵʽ��
CH4������
| 8 |
| 3 |
1
| 8 |
| 3 |
5.60��107L��75% X
X=1.12��108L��
�ʴ�Ϊ��1.12��108��
��4�����صĽṹ��ʽΪ��
 ������˫����������ͬ���칹������ʣ����Ըı�̼��˫�������ӵ�ԭ���Ż�ԭ��д����ͬ���칹��Ϊ��
������˫����������ͬ���칹������ʣ����Ըı�̼��˫�������ӵ�ԭ���Ż�ԭ��д����ͬ���칹��Ϊ�� ��
��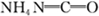 ��
���ʴ�Ϊ��
 ��
��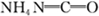 ��
�����������⿼�����Ȼ�ѧ����ʽ����д�������Ȼ�ѧ����ʽ�ļ���Ӧ�ã����ʽṹ�ķ����жϣ�ͬ���칹����д��ԭ�����Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
����[CO(NH2)2]��һ�ַdz���Ҫ�ĸߵ�����,����Ȼ������H2S)Ϊԭ�� �ϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ijЩת������δ�г�����

��ش��������⣺
��1����Ӧ�ٵ����ӷ���ʽ��______________
��2����Ȼ������������Fe2S3��H2O��02��Ӧ�Ļ�ѧ����ʽ��_______��
��3����Ӧ���Ƿ��ȷ�Ӧ���¶����ߣ��÷�Ӧ��ƽ�ⳣ��_______ (�� ����������С�� ���䡱)��H2NCOONH4 (��������泥��Ǻϳ����ص��м��壬����̼ԭ�ӵ��ӻ����������_______�ӻ���
��4����������������������ɫ��ѧ���գ������� 120t������������Ҫ CH4___m3 (��״������
��5����ѧ�������о����ض���ȼ�ϵ�أ���ҺҲ�ܷ��磡�����ֵ��ֱ��ȥ�����з�ˮ�е����أ����ܲ���������ˮ���ܷ��硣����ȼ�ϵ�ؽṹ��ͼ��ʾ������ʱ�����ĵ缫��ӦʽΪ__________