��Ŀ����
4���ش��������⣨����ţ�����1�����������У���©����������ƿ����������ƿ������ƽ���ݷ�Һ©��������Ͳ����ȼ�ճף����������ʷ�����Ǣ٢ۢݣ����и������ʷе㲻ͬ���������ʵ������Ǣۣ����������д��
��2��������NaOH��������500mL 0.2mol/L��NaOH��Һ������������գ�
A�����Ƹ���ҺӦѡ��500 mL����ƿ��
B����������ƽ��ȡ2.0 g����NaOH��
C�����ƺõ�NaOH�������500mL�Ĵ��ձ��У�����Լ250mL����ˮ���ò�������������ȫ�ܽ⣮����ȴ�����º��ձ��е���Һ�ò���������ת��������ƿ��
D������������ˮϴ���ձ�2-3�Σ�����ÿ��ϴ�ӵ���Һ��ע������ƿ������ζ�����ƿ��ʹ��Һ��;��ȣ�
E��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ1-2����ʱ�����ý�ͷ�ι� �μ�����ˮ��Һ����̶������У��Ǻ�ƿ����ҡ�ȣ������ˮʱҺ�泬���̶��ߣ���ʹ��õ���ҺŨ��ƫ�ͣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
F�����ƺõ���Һ���ܣ���ܡ����ܡ������ڴ��������ƿ�У�
���� ��1��ʵ���ҳ����ڷ���IJ����й��ˡ�������������ȡ����Һ�ȣ����õ���������ͨ©������Һ©����������ƿ������������������ʷе㲻ͬ���������ʣ��Դ������
��2��A������������Һ�����ѡȡ����ƿ������ƿ�Ĺ��Ӧ�Դ��ڻ����������Һ�������
B������m=nM=CVM�����Ȼ��Ƶ�������
C�����ݲ����������÷�����
E�����ݶ��ݵIJ��������������ݸò����Ƿ��������Һ��Ũ����Ӱ�������
F����������ƿ�Ĺ�����������
��� �⣺��1����©�������ڹ��ˣ������������Һ����
������ƿ��������һ�����ʵ���Ũ�ȵ���Һ�������������������ʵķ��룻
��������ƿ�����ڷ���е㲻ͬ��Һ�����
����ƽ�����ڳ��������������һ�㲻�������ʵķ��룻
�ݷ�Һ©�������ڷ��뻥�����ܵ�Һ�����
����Ͳ������ȡҺ�壬һ�㲻�������ʵķ��룻
��ȼ�ճ׳�����ȼ�����ʣ�
���������ʷ�����Ǣ٢ۢݣ�ֻ�Т�����������ʷе㲻ͬ���������ʣ�
�ʴ�Ϊ���٢ۢݣ��ۣ�
��2��A��������Һ�������450mL��������ƿ�Ĺ��û��450mL�ģ���500mL�ģ�����ѡȡ����ƿ�Ĺ��Ӧ�Դ���450mL����ѡ500mL������ƿ���ʴ�Ϊ��500��
B��m=nM=CVM=0.2mol/L��0.5L��40g/mol=4.0g��
�ʴ�Ϊ��4.0��
C�������ܽ�ʱҪ�ò��������裬�Լ����ܽ⣬�ʴ�Ϊ����������
E�����ݣ�������ƿ��С�ļ�������ˮ��Һ��ӽ��̶�1��2�������ý�ͷ�ιܵμӣ���ҺҺ��ǡ����̶������У������ˮʱҺ�泬���̶��ߣ�����Һ���ƫ��Ũ��ƫ�ͣ�
�ʴ�Ϊ����ͷ�ιܣ�ƫ�ͣ�
F������ƿֻ��������Һ�����ƣ����ܴ�����Һ���ʴ�Ϊ�����ܣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע������ƿ����ѡȡ������������Һ�����ѡȡ����ƿ������ƿ�Ĺ��Ӧ�Դ��ڻ����������Һ�������

| A�� | ��ͬ��ͬѹ�£�22.4L���κ����嶼��1 mol | |
| B�� | 1molCaCl2��ȫ����ˮ���Եõ�1 mol Ca2+��2 mol Cl- | |
| C�� | �ڱ�״���£�11.2 Lij���������Ϊ22 g������������Է���������44 g/mol | |
| D�� | ��80g NaOH��������1 Lˮ�У�������Һ��NaOH�����ʵ���Ũ����2 mol/L |
| A�� | 1��1 | B�� | 3��2 | C�� | 2��3 | D�� | 2��1 |
| ������ | K+ Na+ Cu+ Al+ |
| ������ | SO${\;}_{4}^{2-}$ HCO${\;}_{3}^{-}$ NO${\;}_{3}^{-}$ OH- |
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��cΪ��ɫ������ɫ�겣������
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�ж��ܲ�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɣ�
��������ʵ����գ�
��1��д��B��D�Ļ�ѧʽ��BKNO3��DCuSO4��
��2������1 mol A����Һ�뺬l molE����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪNa2CO3��
��3����A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ2HCO3-+Ca2++2OH-=CaCO3��+CO32-+H2O��
��4��C��������ˮ���������ӷ���ʽ���ʵ�����˵���侻ˮԭ��Al3++3H2O?Al��OH��3�����壩+3H+ˮ�����ɵ�������������������ˮ�е������ᄏˮ
��5����������lmol��C��Һ����μ���Ba��OH��2��Һ�����ɳ����������Ϊ466g��
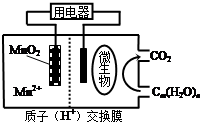
| A�� | �������ΪCm ��H2O��n������һ�������� | |
| B�� | ������ӦʽΪ��MnO2+4H++2e-=Mn2++2H2 O | |
| C�� | �ŵ�����У�H+�������������� | |
| D�� | ��Cm ��H2O��n�������ǣ�����·��ת����6NA����ʱ����Ӧ��������������60g |
| A�� | �������������ƽ��Ħ����������ʱ����Ӧ�ﵽ��ƽ�� | |
| B�� | ������������ѹǿ����ʱ����Ӧ�ﵽƽ�� | |
| C�� | �����¶ȣ�ƽ�������ƶ� | |
| D�� | ƽ������X��������Ӧ�ġ�H���� |
| A�� | 3��1 | B�� | 1��3 | C�� | 2��3 | D�� | 1��2 |
| A�� | AlCl3 | B�� | KHCO3 | C�� | Fe2��SO4��3 | D�� | NH4HCO3 |
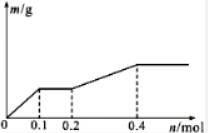 ��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������
��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������| A�� | Ag+��X3+��Cu2+��H+��X2+ | B�� | Ag+��Cu2+��X3+��H+��X2+ | ||
| C�� | Cu2+��X3+��Ag+��X2+��H+ | D�� | Cu2+��Ag+��X3+��H+��X2+ |