��Ŀ����
�����������ϣ��ش��������⣺
(1)H2O2�ĽṹʽΪ________����������Ԫ�صĻ��ϼ�Ϊ ________��
(2)H2O2�Ľṹ�ص������H2O2���ж������ʡ�
��˫��ˮ�ж������ʣ�Na2O2��2HCl===H2O2��2NaCl�������Ӧ������˫��ˮ��________�ԡ�
�����з�̪��NaOH��Һ�еμ�H2O2����Һ��ɫ��ȥ��������H2O2��________�ԡ�
�۽�H2O2���뾭�ữ��KMnO4��Һ�У���Һ���Ϻ�ɫ��ʧ����������H2O2��________��
(3)H2O2����Ϊ��ɫ��������������____________________________��
(4)�������ʲ���ʹ��̼����ʧЧ����________(��д��ĸ���)��
A��MnO2 B. H2S C��CH3COOH D. NaHCO3
(5)H2O2���ȶ����ֽ⣬�ڴ��������·ֽ���졣
��ʵ������H2O2��O2�Ļ�ѧ����ʽΪ��_______________________________��
����֪���з�Ӧ��һ�������¿��Է�����
a��H2O2��2Fe2����2H��===2Fe3����2H2O�� b��H2O2��2Fe3��===2Fe2����O2����2H���������Ϸ�Ӧ��Fe2��ʵ��������________���ã��ܷ�ӦʽΪ__________________________��
(2)���������(Ư��)���ۻ�ԭ
(3)H2O2��������ʱ�IJ�����H2O������Ⱦ����
(4)D
(5)��2H2O2
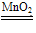 2H2O��O2�����ڴ���(���)��2H2O2
2H2O��O2�����ڴ���(���)��2H2O2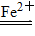 2H2O��O2��
2H2O��O2�� 
| |||||||||||||||||||||||||||||
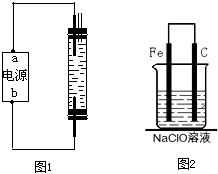 ��2010?��̨һģ��2008��5��12���ҹ��Ĵ��봨�����ش����Ϊ��ֹ�ڴ���֮���߲����У�ȫ�����������������˴����ĸ�������Һ����NaClO��Һ��ijУ̽����ѧϰС�������Һ�������ƣ�NaClO�����Ʊ������ʵȽ�����̽����
��2010?��̨һģ��2008��5��12���ҹ��Ĵ��봨�����ش����Ϊ��ֹ�ڴ���֮���߲����У�ȫ�����������������˴����ĸ�������Һ����NaClO��Һ��ijУ̽����ѧϰС�������Һ�������ƣ�NaClO�����Ʊ������ʵȽ�����̽����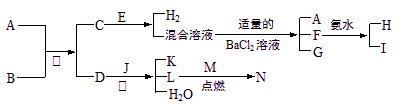 A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ
A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ