��Ŀ����
ij��ѧ��ȤС����ֻ������������ͭ�Ĺ�ҵ������ȡ�������Ȼ�����Һ���̷�����(FeSO4��7H2O)�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
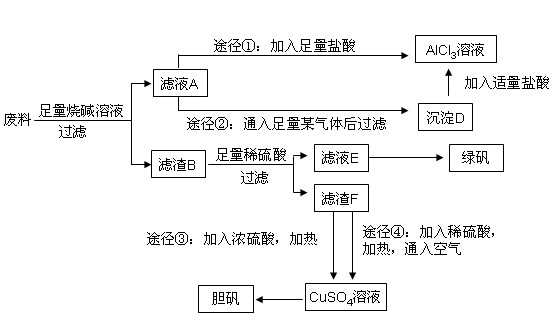
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ�� ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�;������ͨ���ij���壨��̬ʱ�������˹����꣩��д��������ĵ���ʽ ������Ϊ�Ϻ�����;���� ����ٻ�ڣ��������ǣ� ��
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���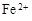 ��
��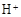 �⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
�⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
��4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ� �� ��
��5��;���ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ�� ����Ȼ���

��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ�� ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�;������ͨ���ij���壨��̬ʱ�������˹����꣩��д��������ĵ���ʽ ������Ϊ�Ϻ�����;���� ����ٻ�ڣ��������ǣ� ��
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���
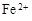 ��
��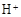 �⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
�⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ����4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ� �� ��
��5��;���ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ�� ����Ȼ���
��1��2Al +2OH�D + 2H2O = 2AlO2�D +3H2�� (2��)
��2��
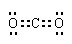 (1��) �� (1��) ;������ȡ��AlCl3��Һ�л���NaCl����(1��)
(1��) �� (1��) ;������ȡ��AlCl3��Һ�л���NaCl����(1��)��3��Fe3�� (1��) ȡ�����μ�KSCN������Ѫ��ɫ������Fe3�� ��(1��)
��4���ɱ��� (1�֣��������֣���ͬ) ���������ж����� (1��)
��5��2Cu +2H2SO4 +O2�� 2CuSO4 +2H2O (2��)
��6������ϴ�� (1��)
���������
��1����������ֻͭ�������ռ���Һ��Ӧ��
��2����NaAlO2��Ӧ����Al(OH)3����̬ʱ�������˹����꣬������CO2 ��NaAlO2 +4HCl=AlCl3+NaCl+2H2O ����AlCl3��Һ�л���NaCl���ʡ�
��3��Fe2��������������Fe3����Fe3����KSCN��Ӧ����Ѫ��ɫ��Fe2�����ܡ�
��4��;����Cu+2H2SO4= CuSO4+ SO2��+2H2O
;���� 2Cu +2H2SO4 +O2 = 2CuSO4 +2H2O;������ϡH2SO4�ɱ��ͣ��������ж�����

��ϰ��ϵ�д�
�����Ŀ
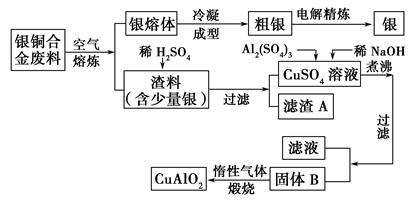
 ____CuAlO2��________����
____CuAlO2��________����